Figure 2
- ID
- ZDB-FIG-220302-178
- Publication
- Schouten et al., 2022 - Stapling of Peptides Potentiates the Antibiotic Treatment of Acinetobacter baumannii In Vivo
- Other Figures
- All Figure Page
- Back to All Figure Page
|
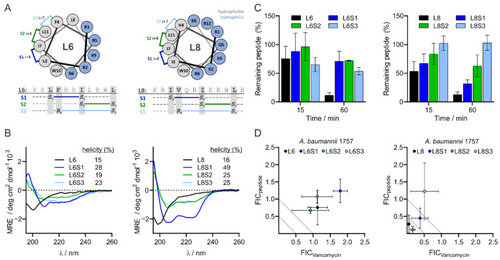
Design and properties of L6 and L8 as well as stapled versions. Design and structure of stapled L6 and stapled L8 (A). The degree of α-helicity of L6, L8 and the stapled variants of the peptides was determined with CD spectroscopy. Relative helicity was calculated using circular dichroism analysis using neural networks (CDNN) software (B). Stability of L6, L8 and the stapled variants of the peptides in human serum were analyzed by LCMS and quantified using total ion count (TIC) of selected molecular ions (C). MIC values of the stapled peptides or vancomycin alone and MIC values of the stapled peptides combined with vancomycin or vancomycin in combination with the stapled peptides against Gram-negative bacteria were determined via checkerboard assay. The FIC values were defined as the ratio of either the MIC value of the stapled peptide in combination with vancomycin over the MIC value of the stapled peptide alone (FICL6 and FICL8), or the ratio of the MIC value of vancomycin combined with the stapled peptide over the MIC value of vancomycin alone (FICVancomycin) (D). The data are presented as mean ± standard deviation from three independent experiments. |