Figure 3
- ID
- ZDB-FIG-201130-29
- Publication
- Muñoz-Sánchez et al., 2020 - Autophagy and Lc3-Associated Phagocytosis in Zebrafish Models of Bacterial Infections
- Other Figures
- All Figure Page
- Back to All Figure Page
|
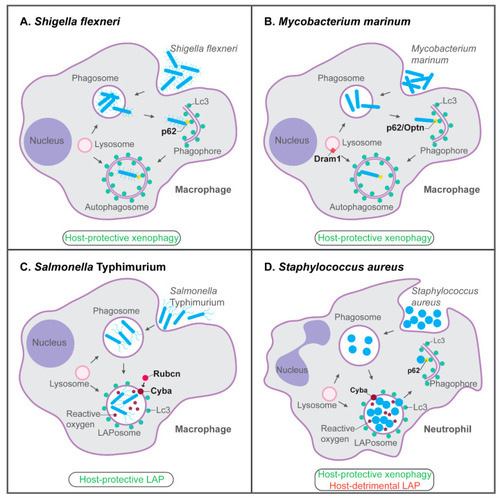
Host-protective and host-detrimental interactions of bacterial pathogens with the autophagy machinery in zebrafish infection models. ( |