Figure 1
- ID
- ZDB-FIG-200306-69
- Publication
- Laselva et al., 2019 - Activity of lumacaftor is not conserved in zebrafish Cftr bearing the major cystic fibrosis-causing mutation
- Other Figures
- All Figure Page
- Back to All Figure Page
|
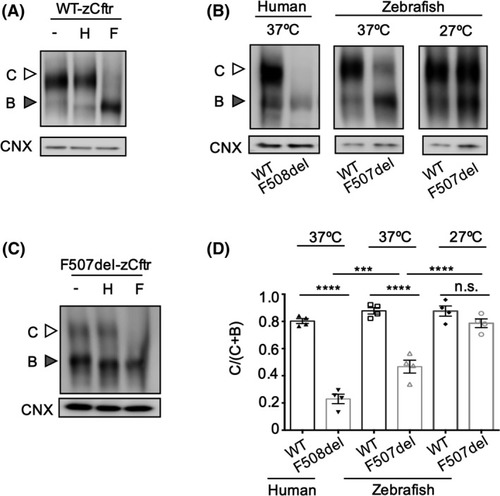
The processing defect of F507‐zCftr is fully rescued by low temperature correction at 27°C. A, HEK293 cells were transiently transfected with WT‐zCftr‐GFP. To evaluate glycosylation status, immunoblots show WT‐zCftr‐GFP control (−) and its sensitivity to endoglycosidase H (H) and peptide‐N‐glycosidase F (F). White arrowhead, complex glycosylated (Band C); gray arrowhead, core glycosylated (Band B). The zCftr‐GFP was detected with an antibody against GFP. B, HEK293 cells were transiently transfected with WT‐hCFTR‐GFP, F508del‐hCFTR‐GFP, WT‐zCftr‐GFP, or F507del‐zCftr‐GFP at 37°C (left) or 27°C (right) (n = 4). The zebrafish mutant recapitulates the primary defect in processing observed in the human mutant at 37°C. C, Immunoblots show F507del‐zCftr‐GFP protein exhibit partial processing to mature form, Band C at 37ºC, as evident in its resistance to endoglycosidase H (H) and sensitivity to peptide‐N‐glycosidase F (F). D, Bar graphs show the mean (±SEM) of the ratio (Band C)/(Band (C + B)) of the WT and F508del‐hCFTR‐GFP proteins plus WT and F507del‐zCftr‐GFP protein at 37 and 27°C (n = 4) (*** |