Figure 4 - figure supplement 3
- ID
- ZDB-FIG-190723-955
- Publication
- Hardy et al., 2019 - Detailed analysis of chick optic fissure closure reveals Netrin-1 as an essential mediator of epithelial fusion
- Other Figures
- All Figure Page
- Back to All Figure Page
|
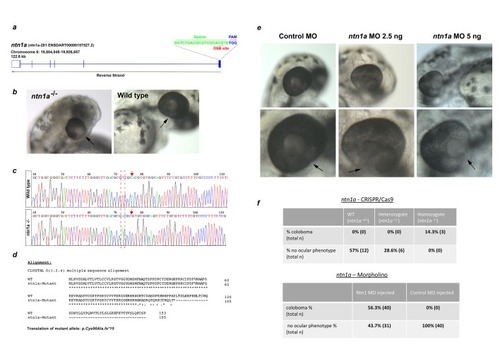
Gross ocular phenotype analyses of ntn1-deficient zebrafish. ( |
| Fish: | |
|---|---|
| Knockdown Reagent: | |
| Observed In: | |
| Stage: | Long-pec |