Fig. S3
- ID
- ZDB-FIG-121101-30
- Publication
- Cox et al., 2012 - An essential role of variant histone h3.3 for ectomesenchyme potential of the cranial neural crest
- Other Figures
- All Figure Page
- Back to All Figure Page
|
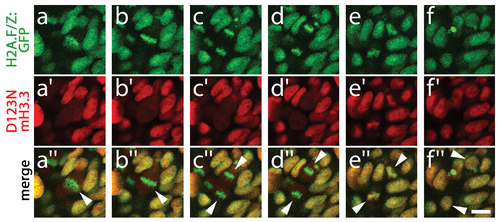
D123N H3.3 protein remains stable during mitosis. Time course of confocal images from H2A.F/Z:GFP embryos expressing D123N mCherry(m)H3.3 fusion protein, showing a cell (arrowhead in merged image a′′) progressing through the stages of mitosis including metaphase (a/a′/a′′ and b/b′/b′′), anaphase (c/c′/c′′ and d/d′/d′′), telophase (e/e′/e′′) and the eventual establishment of two new daughter cells (arrowheads, f/f′/f′′). a–d, H2A.F/Z:GFP localizes to condensed chromosomes during both metaphase and anaphase. a2–d2, In contrast, D123N mCherry-H3.3 fails to co-localize with H2A.F/Z:GFP and appears as a weak diffuse signal throughout the cell(s) after nuclear envelope breakdown. e/e′/e′′, H2A.F/Z:GFP and D123N mCherry-H3.3 subsequently become co-localized during the re-establishment of the nuclear membranes during telophase. f/f′/f′′, Nuclear co-localization continues into interphase in both daughter cells. The rapid re-appearance of strong nuclear mCherry-H3.3 signal in telophase (16/16 cells over 2 embryos) confirms that the low-level diffuse D123N mCherry-H3.3 signal observed during metaphase/anaphase results from a failure to localize to condensed chromosomes rather than protein degradation. Scale bar = 10 μm. |