Fig. S3
- ID
- ZDB-FIG-091217-129
- Publication
- Edeling et al., 2009 - Structural requirements for PACSIN/Syndapin operation during zebrafish embryonic notochord development
- Other Figures
- All Figure Page
- Back to All Figure Page
|
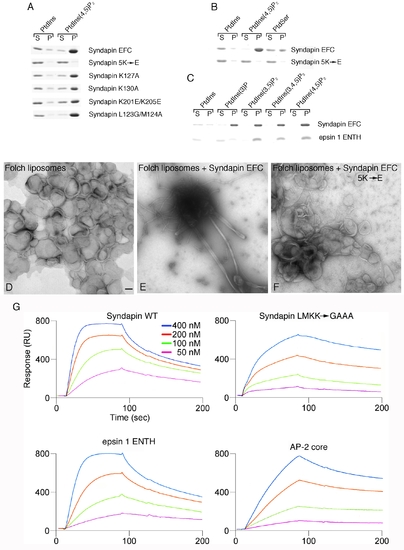
Syndapin EFC domain-liposome interactions. (A) Syndapin EFC domain mutant binding to synthetic liposomes. Coomassie-blue stained gels of aliquots of supernatant (S) and pellet (P) fractions from sedimentation assays are shown. (B) Syndapin EFC domain binding to PtdSer containing liposomes. A three-times excess of PtdSer (30%) is bound less effectively than are PtdIns(4,5)P2 (10%) containing liposomes. (C) The syndapin EFC domain binds to a similar extent to PtdIns(3)P, PtdIns(3,5)P2, PtdIns(4,5)P2 and PtdIns(3,4,5)P3, but the epsin 1 ENTH domain does not bind PtdIns(3)P. This indicates that the interaction of the Syndapin EFC domain with liposomes is largely via general electrostatics and not strongly stereospecific. (D-F) Negatively-stained transmission electron micrographs of liposome tubulation assays with the Syndapin EFC domain. Folch lipid liposomes alone (panel D), Folch liposomes plus the Syndapin EFC domain (panel E), Folch liposomes plus Syndapin EFC 5K→E mutant (panel F). Scale bar = 100 nm. Notice that the wild-type Syndapin EFC domain generates both broad (~ 80 nm) and narrow (~ 20 nm) diameter tubules as well as low levels of small spherical structures that appear to be vesicles. (G) Sensogram traces from assays using PtdIns(4,5)P2-containing 200 nm synthetic liposomes immobilized on an L1 chip. The indicated concentration of the Drosophila Syndapin EFC domain (1-304; WT), Syndapin (1-304) LMKK→GAAA mutant, epsin 1 ENTH domain or AP-2 core were flowed over the liposomes followed by washing. The derived equilibrium dissociation constant (KD) values are: 88 nM for the wild-type Syndapin EFC domain, 1.2 μM for the Syndapin LMKK→GAAA mutant, 590 nM for the epsin 1 ENTH domain and 7.3 μM for the heterotetrameric AP-2 core. |