- Title
-
The cytoskeleton adaptor protein Sorbs1 controls the development of lymphatic and venous vessels in zebrafish
- Authors
- Veloso, A., Bleuart, A., Conrard, L., Orban, T., Bruyr, J., Cabochette, P., Germano, R.F.V., Schevenels, G., Bernard, A., Zindy, E., Demeyer, S., Vanhollebeke, B., Dequiedt, F., Martin, M.
- Source
- Full text @ BMC Biol.
|
|
|
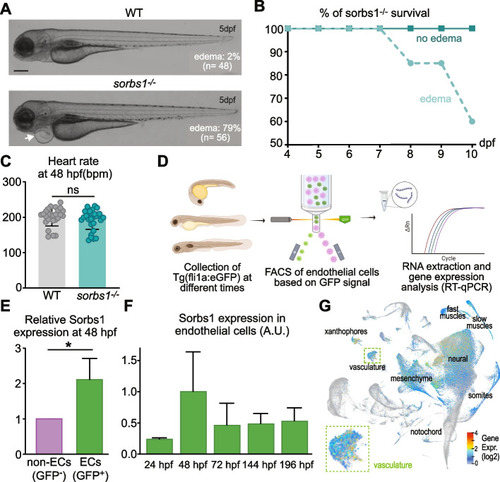
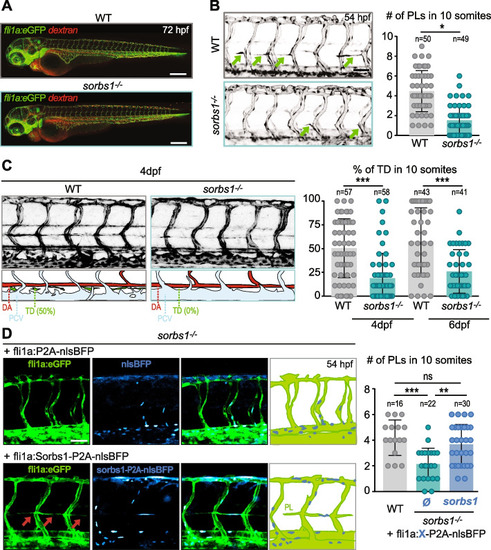
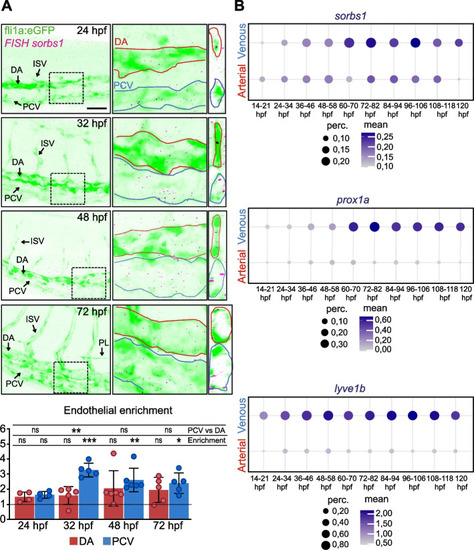
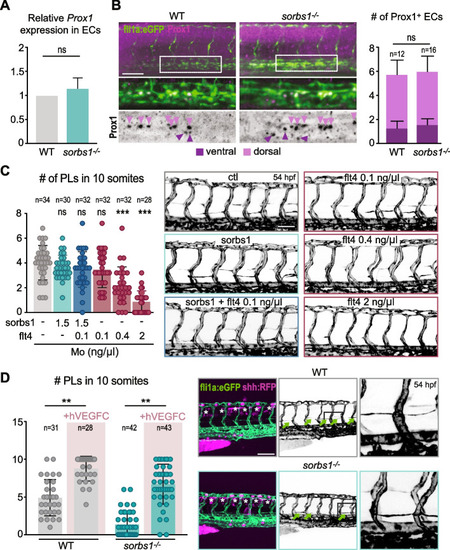
Sorbs1 is necessary for trunk lymphangiogenesis in vivo. |
|
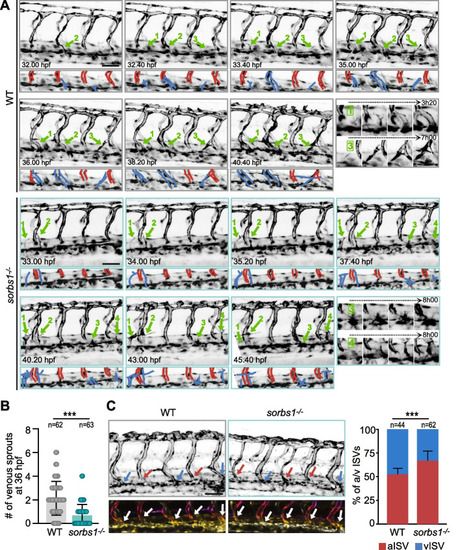
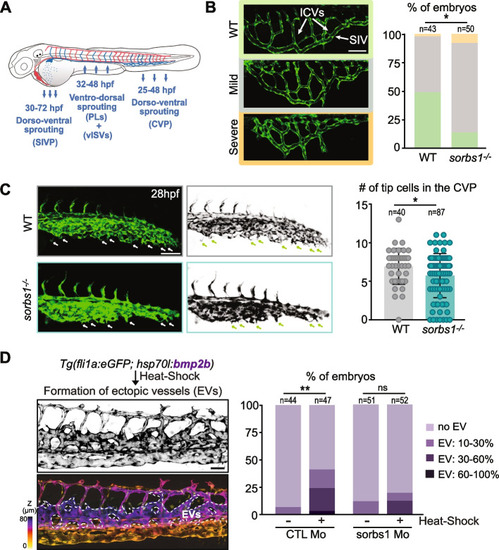
Secondary sprouting is impaired in the absence of |
|
|
|
Sorbs1 functions independently of Vegfc signaling during in vivo lymphangiogenesis. |
|
Sorbs1 depletion results in defects in ventral sprouting from the PCV. |
|
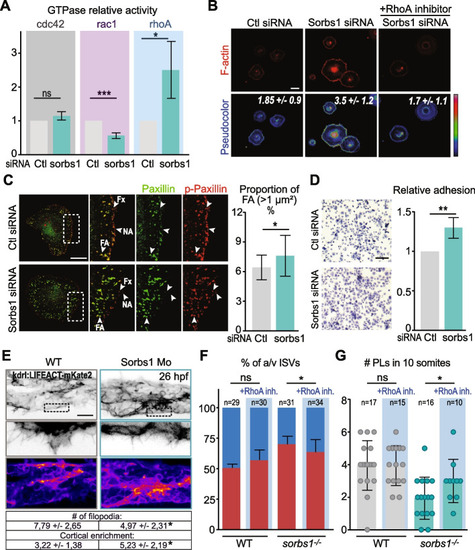
Sorbs1 controls EC adhesive properties via RhoGTPases in vitro and in vivo. |