- Title
-
Obscurin Maintains Myofiber Identity in Extraocular Muscles
- Authors
- Kahsay, A., Dennhag, N., Liu, J.X., Nord, H., Rönnbäck, H., Thorell, A.E., von Hofsten, J., Pedrosa Domellöf, F.
- Source
- Full text @ Invest. Ophthalmol. Vis. Sci.
|
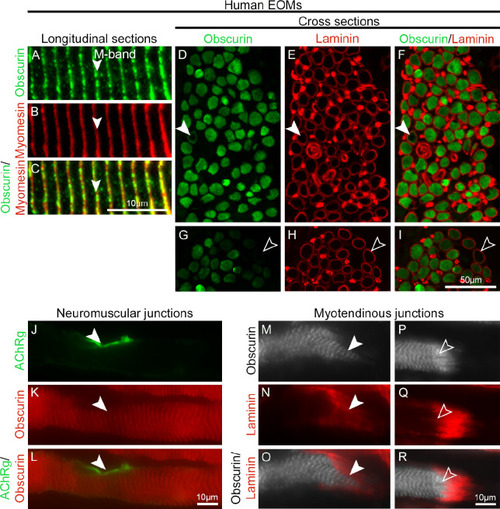
Localization and distribution of obscurin in human EOMs. ( |
|
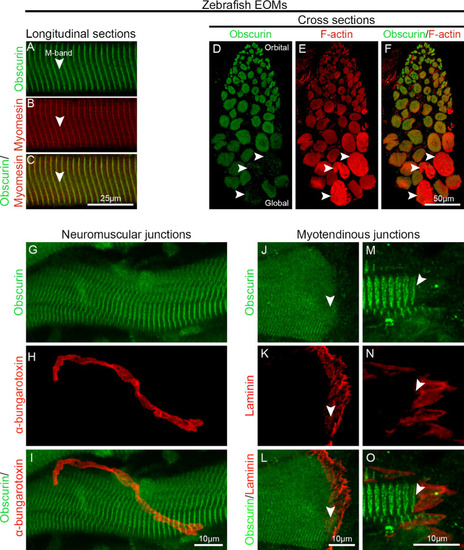
Obscurin distribution in zebrafish EOMs. ( |
|
|
|
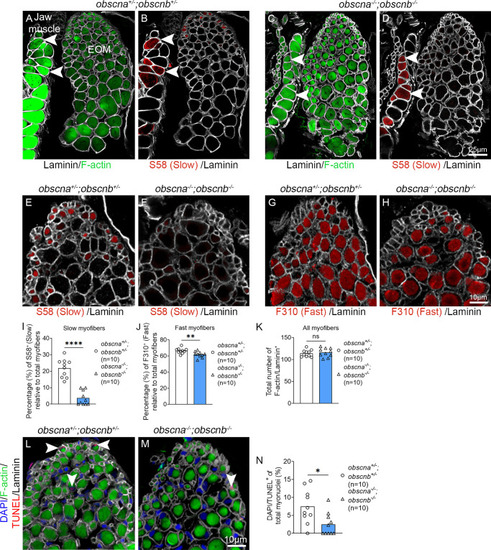
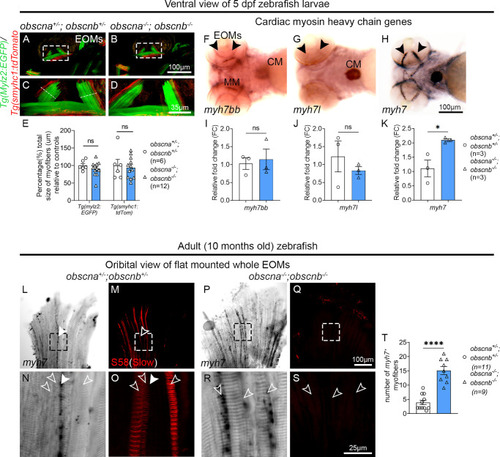
Quantification of slow and fast myofibers in the EOMs of adult obscurin mutants and sibling controls. Cross-sections of 10-month-old adult zebrafish EOMs immunolabeled with phalloidin to identify all myofibers (labeling of F-actin by phalloidin, |
|
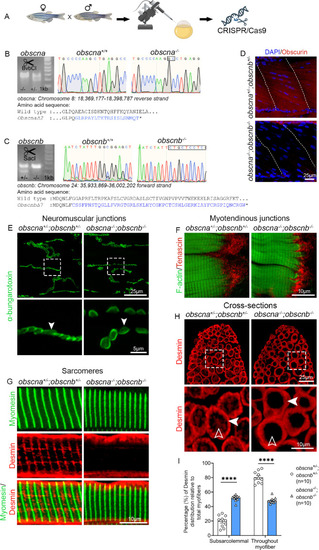
Expression of |
|
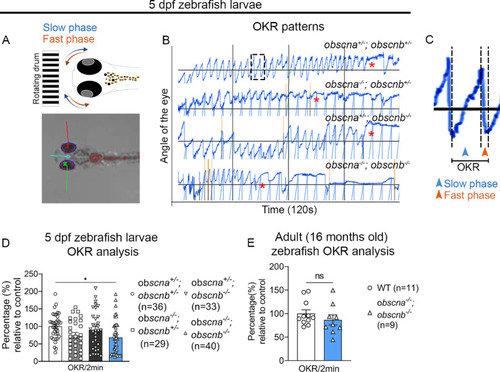
Optokinetic response analysis of the zebrafish EOMs: ( |