- Title
-
Kif21a deficiency leads to impaired glomerular filtration barrier function
- Authors
- Riedmann, H., Kayser, S., Helmstädter, M., Epting, D., Bergmann, C.
- Source
- Full text @ Sci. Rep.
|
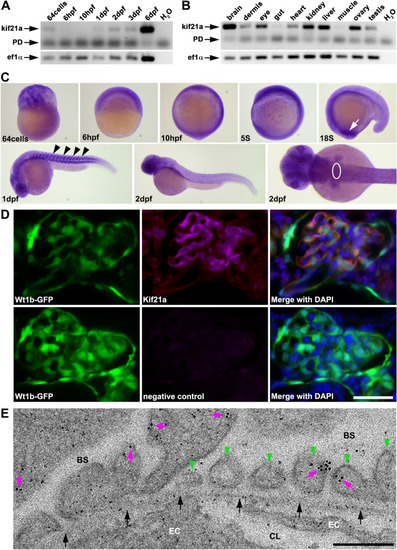
Kif21a shows a specific expression during zebrafish development and localized to the glomerular filtration barrier. ( |
|
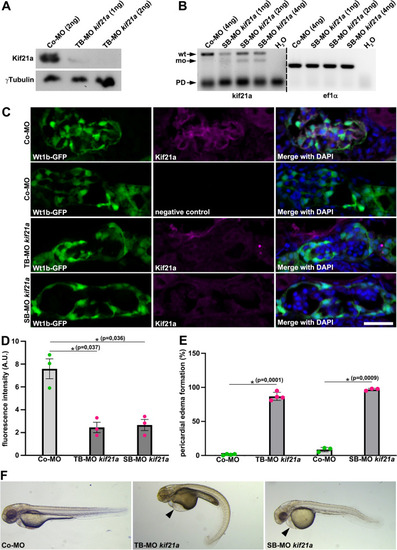
Kif21a deficiency results in pericardial edema formation and reduced glomerular Kif21a signal in zebrafish. ( |
|
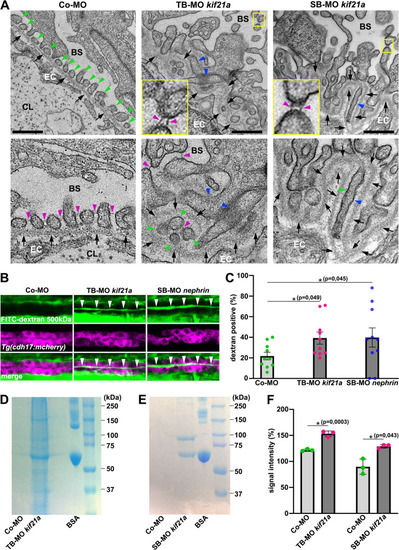
Kif21a deficiency results in defective podocyte morphology leading in a leaky glomerular filtration barrier and proteinuria. ( |