- Title
-
The proneural factors Ascl1a and Ascl1b contribute to the terminal differentiation of dopaminergic GABAergic dual transmitter neurons in zebrafish
- Authors
- Altbürger, C., Rath, M., Wehrle, J., Driever, W.
- Source
- Full text @ Dev. Biol.
|
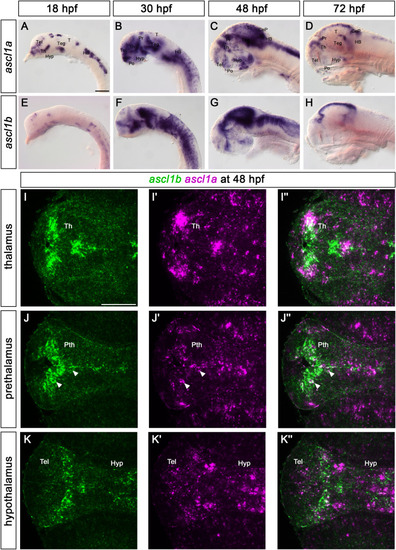
The expression of ascl1a and ascl1b throughout development. (A–H) Whole-mount in situ hybridizations for ascl1a (A–D) and ascl1b (E–H). Lateral views of wildtype embryos at the indicated stages. Anterior is to the left. (I–K″) Fluorescent whole mount in situ hybridization of ascl1a and ascl1b at 48 hpf. Dorsal views of single confocal planes of wildtype embryos. Anterior is to the left. The expression of ascl1a and ascl1b is mainly exclusive in the thalamus (I–I″) and hypothalamus (K–K″). In the prethalamus (J-J″) a few cells lining or being close to the ventricle express both paralogs (white arrowheads). The confocal stack is provided as Supplemental Video S1. Abbreviations: CeP, cerebellar plate; HB, hindbrain; Hyp, hypothalamus; Po, preoptic region; Pr, pretectum; Pth, prethalamus; T, tectum; Teg, tegmentum; Tel, telencephalon; Th, thalamus. Scale bars: 100 μm. |
|
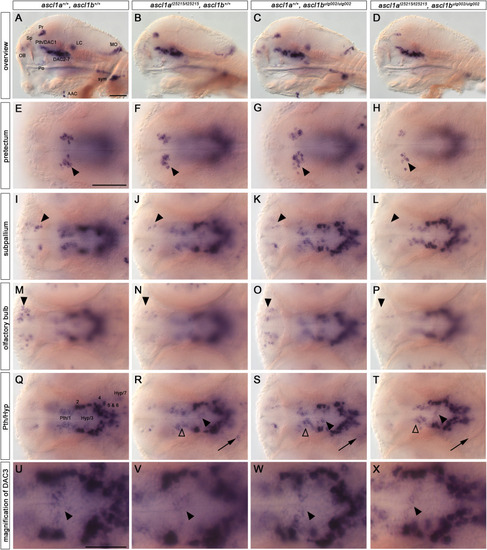
Catecholaminergic neurons are reduced in ascl1a, ascl1b double mutants. (A–X) th WISH of 96 hpf embryos. Lateral views (A–D) and dorsal views (E–Y) of wildtype, single ascl1a and ascl1b mutant, and double mutant embryos. Anterior is to the left. Double mutant embryos (D) show a severe reduction or absence of NA cell groups (AAC, LC, MO, sym) when compared to wildtype or single mutant embryos. The LC NA neurons are also lost in ascl1a single mutants (B). Pretectal neurons (E–H) are strongly reduced in double mutants (arrowheads) in comparison to wildtype. Subpallial DA neurons (I–L) are reduced in single and double mutants (arrowheads) compared to wildtype with the strongest reduction in double mutants. Olfactory bulb neurons (M–P) are reduced in ascl1a single mutants and double mutants (arrowheads). In the prethalamus and hypothalamus (Q–T) only DAC1 (non-filled arrowheads), DAC (filled arrowheads) and DAC7 (arrows) are reduced in double mutants, whereas DAC2, 4, 5 and 6 are not affected. The magnification of cluster DAC3 (U-X, from overviews Q-T) highlights the observed reduction (arrowheads). For numbers of analyzed embryos see Supplemental Table S2. Abbreviations: AAC, arch associated cluster; DAC, DA cluster; Hyp, hypothalamus; LC, locus coeruleus; MO, medulla oblongata; OB, olfactory bulb; Po, preoptic region; Pr, pretectum; Pth, prethalamus; Sp, subpallium; sym, sympathetic neurons. Scale bars: 50 μm in A for A-A and in E for E-T; 25 μm in U for U-X. |
|
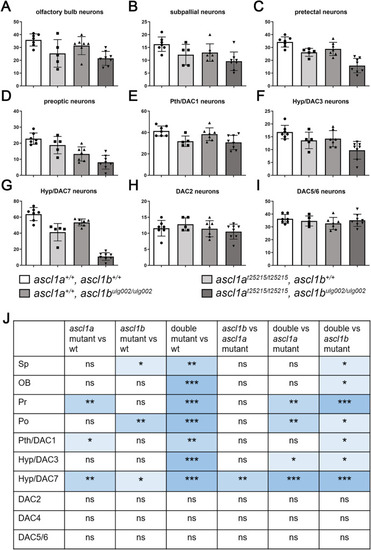
Absence of Ascl1 activity leads to a significant decrease of dopaminergic neurons. (A–I) Cell counts (DAC4 data not shown) and (J) statistical analysis of the immunofluorescence staining shown in Fig. S1. Neuron numbers of different genotypes were compared using the Wilcoxon-Mann-Whitney rank-sum non-parametric test. P-values indicate significance if they are <0.05 (**, 0.001–0.01; ***, 0.0001–0.001). For numbers of analyzed embryos see Supplemental Table S2. Abbreviations: DAC, dopaminergic cluster; Hyp, hypothalamus; ns, not significant; OB, olfactory bulb; Po, preoptic region; Pr, pretectum; Pth, prethalamus; Sp, subpallium. |
|
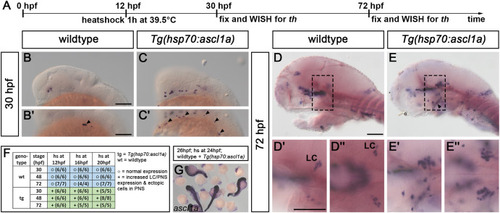
Global overexpression of ascl1a induces supernumerary and ectopic th+ neurons. (A) Experimental timeline of heatshock overexpression experiments. Embryos from a Tg(hsp70:ascl1a) outcross were heat shocked at 12 hpf and fixed at 30 or 72 hpf for analysis (A, B-E″). (B–E) Whole-mount in situ hybridization for th (B-E″) and ascl1a (G), stages as indicated. Dorsal views (D″,E″) and lateral views (B,B′,C,C′,D,D′,E,E′), genotypes as indicated. (G) Heat shock leads to a global overexpression of ascl1a in Tg(hsp70:ascl1a) outcrosses. (B–C′) Tg(hsp70:ascl1a)+ embryos have ectopic th+ neurons in the peripheral nervous system (arrowheads) when compared to wildtype siblings at 30 hpf. (D-E″) At 72 hpf Tg(hsp70:ascl1a)+ embryos have ectopic th+ neurons in the peripheral nervous system (arrowhead) and additional neurons of the LC. Box with dashed lines in D and E mark magnified part in D′ and E’ (D″ and E’‘: dorsal views of regions corresponding to lateral views in D′ and E′). (F) Summary of the observed phenotypes. For numbers of analyzed embryos see Supplemental Table S2. Abbreviations: hs, heat shock; LC, locus coeruleus; PNS, peripheral nervous system; WISH, whole mount in situ hybridization. Anterior is to the left. Scale bars: 100 μm. |
|
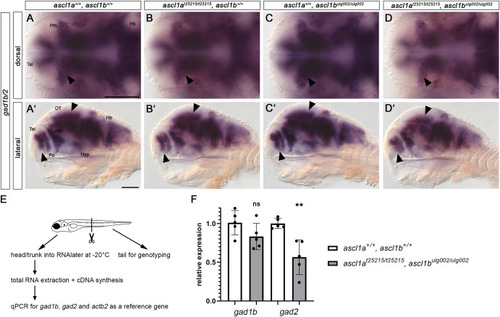
Ascl1 is required for proper GABAergic neurogenesis. (A-D′) Whole-mount in situ hybridization for gad1b/2 on 96 hpf embryos. Dorsal (A–D) and lateral views (A′-D′) of wildtype, single and double mutant ascl1a and ascl1b embryos. Anterior is to the left. Scale bars: 100 μm. Absence of Ascl1 activity leads to a reduction of GABAergic neurons visualized by gad1b/2 expression in the prethalamus, telencephalon and optic tectum (arrowheads, A-D′). In contrast, the expression of gad1b/2 appears normal in the hindbrain in all analyzed genotypes. For numbers of analyzed embryos see Supplemental Table S2. Abbreviations: HB, hindbrain; Hyp, hypothalamus; OT, optic tectum; Po, preoptic region; Pth, prethalamus; Tel, telencephalon. (E,F) qPCR for gad1b and gad2 expression in 96 hpf embryos. (E) 96 hpf embryos were tail-clipped and processed as depicted in the schematic. (F) The expression of gad1b and gad2 is reduced in double mutant embryos compared to wildtype, but only significantly in case of gad2. qPCRs were performed with five biological replicates per genotype, and measurements with three technical replicates, dots show biological replicates. (p = 0.0024; unpaired t-test on ΔCq values). Error bars - standard error of the mean. |
|
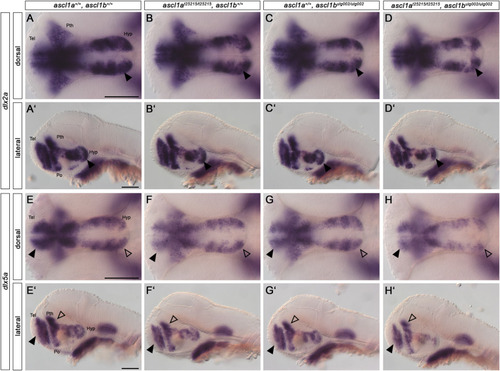
Ascl1 differentially regulates dlx2a and dlx5a genes. (A–H) Whole-mount in situ hybridization for dlx2a (A-D′) and dlx5a (E-H′) at 48 hpf. Dorsal (A–H) and lateral (A′-H′) views, genotypes as indicated. Anterior is to the left. The expression of dlx2a appears largely unchanged in all genotypes except the posterior hypothalamic region (arrowheads) in the double mutant. The expression of dlx5a is reduced in the telencephalon (filled arrowheads), prethalamus (non-filled arrowheads), and in the hypothalamus. For numbers of analyzed embryos see Supplemental Table S2. Abbreviations: Hyp, hypothalamus; Po, preoptic region; Pth, prethalamus; Tel, telencephalon. Scale bars: 100 μm. |
|
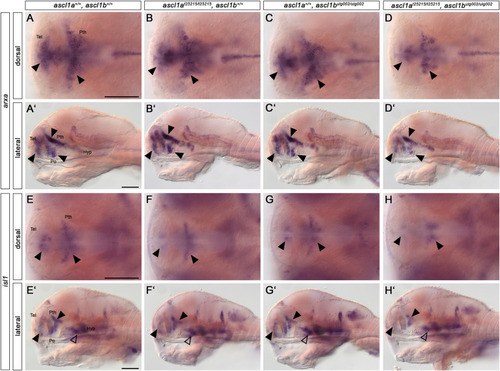
Ascl1 regulates downstream effectors during dopaminergic differentiation. (A-H′) Whole-mount in situ hybridization for arxa (A-D′) and isl1 (E− H′) expression in 96 hpf embryos. Dorsal (A–H) and lateral views (A′-H′) of wildtype, single and double ascl1a and ascl1b mutant embryos. Anterior is to the left. Loss of Ascl1 function leads to a reduction of arxa expression (A-D′) in the telencephalon, prethalamus and preoptic region (arrowheads). The expression of isl1 (E-H′) is reduced in the telencephalon, prethalamus and hypothalamus (arrowheads) in double mutants compared to wildtype and single mutants. For numbers of analyzed embryos see Supplemental Table S2. Abbreviations: Hyp, hypothalamus; Po, preoptic region; Pth, prethalamus; Tel, telencephalon. Scale bars: 100 μm. |
|
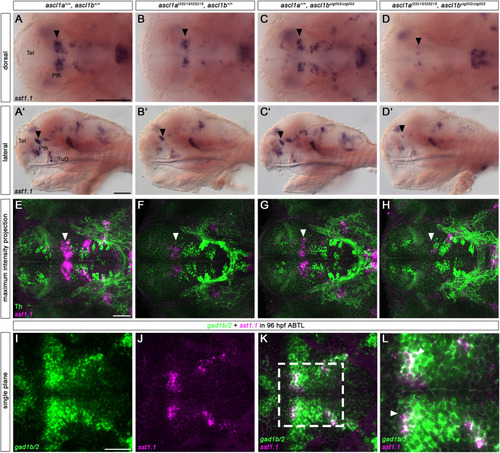
sst1.1+ neurons require Ascl1 function for their proper development. (A-D′) Whole-mount in situ hybridization for sst1.1. Loss of Ascl1 function leads to a severe reduction of sst1.1+ neurons in the prethalamus (A-D′, black arrowheads). A lesser reduction is already detectable in single mutants. ascl1a and double mutants also have less sst1.1+ neurons in the thalamus and the TuD region of the hypothalamus (B,B′,D,D′, non-filled arrowheads). (E–H) Fluorescent sst1.1 in situ hybridization coupled with immunofluorescence for Th. The reduced sst1.1+ neurons (E-H, white arrowheads) do not express Th, but are adjacent to the prethalamic DA neurons, which are also reduced in double mutants. (I–L) Fluorescent in situ hybridization for gad1b/2 and sst1.1. Coexpression analysis with gad1b/2 (I–L) shows that the sst1.1+ neurons in the prethalamus are GABAergic (white arrowheads in L - magnification of the entangled box in K). (A–H) 96 hpf wildtype, single or double ascl1a and ascl1b mutant embryos. (A-D, E-L) Dorsal and (A′-D′) lateral views. (E–H) z-projections of confocal stacks. (I–L) Single confocal planes of a wildtype embryo. Anterior is to the left. For numbers of analyzed embryos see Supplemental Table S2. Abbreviations: Pth, prethalamus; Tel, telencephalon; TuD, tuberal domain, dorsal part. Scale bars: A-D’ - 100 μm, E-K - 50 μm (for scale of L see K). |
|
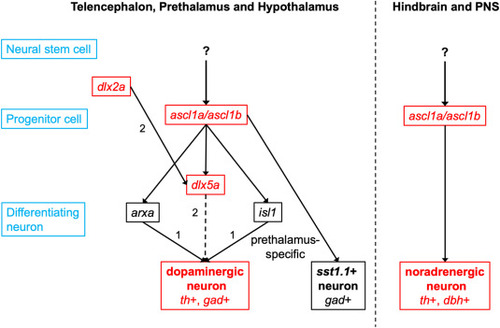
Ascl1 in neurogenesis of GABAergic DA, NA and Sst1.1 dual transmitter neurons Ascl1a and Ascl1b mediate the transition from early (dlx2a+) to late (dlx5a+) GABAergic DA progenitors and the initiation of terminal differentiation. Terminal differentiation is then orchestrated by other transcription factors, for example by Arxa in the prethalamus and preoptic region and by Isl1 in the prethalamus. Ascl1a and Ascl1b are also crucial for the formation of sst1.1 expressing neurons, which are also GABAergic. NA neuron development is strongly dependent on Ascl1a and Ascl1b function. The upstream regulators of ascl1a and ascl1b in DA and NA neurogenesis remain to be identified. The arrows do not indicate exclusive regulation, but genetic interactions. Black gene names indicate pan-GABAergic DA regulators and red gene names prethalamus specific regulators. References: (1) Filippi et al. (2012); (2) Macdonald et al. (2013). |
Reprinted from Developmental Biology, 505, Altbürger, C., Rath, M., Wehrle, J., Driever, W., The proneural factors Ascl1a and Ascl1b contribute to the terminal differentiation of dopaminergic GABAergic dual transmitter neurons in zebrafish, 587458-74, Copyright (2023) with permission from Elsevier. Full text @ Dev. Biol.