- Title
-
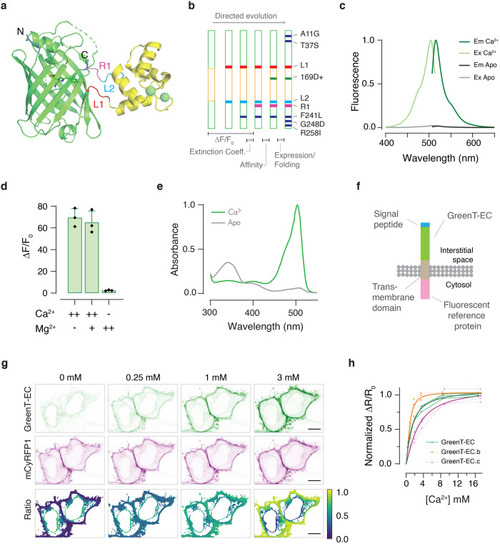
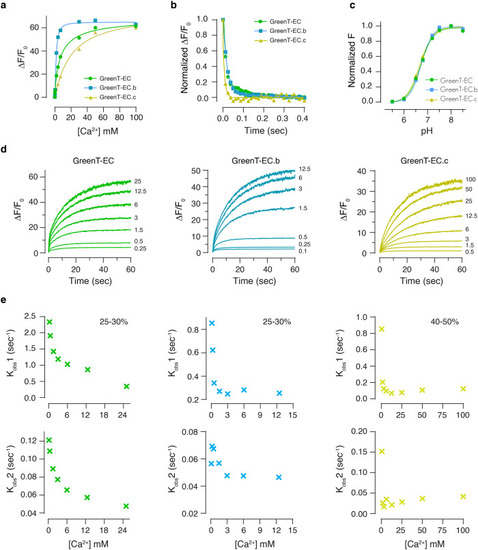
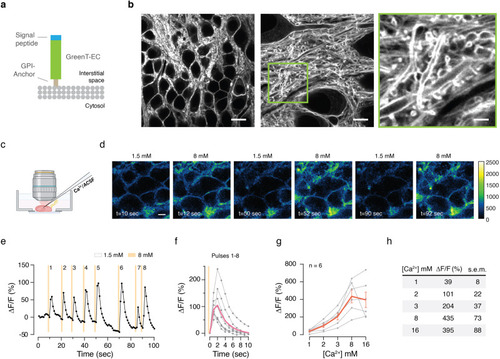
Fluorescent sensors for imaging of interstitial calcium
- Authors
- Valiente-Gabioud, A.A., Garteizgogeascoa Suñer, I., Idziak, A., Fabritius, A., Basquin, J., Angibaud, J., Nägerl, U.V., Singh, S.P., Griesbeck, O.
- Source
- Full text @ Nat. Commun.
|
|
|
|
|
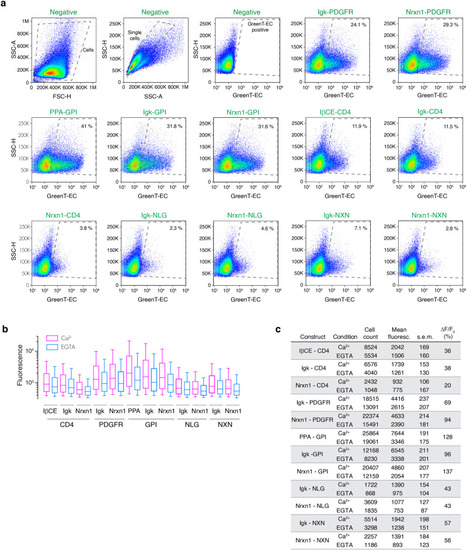
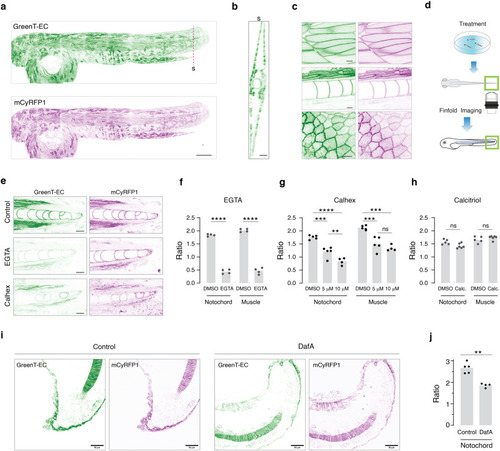
Twelve combinations of export signal peptides and transmembrane/anchoring domains were used to target GreenT-EC to the surface of the cell. HeLa cells were transiently transfected with each construct and analyzed using flow cytometry. |
|
|
|
|
|
|