- Title
-
Optimising the zebrafish Cre/Lox toolbox. Codon improved iCre, new gateway tools, Cre protein and guidelines
- Authors
- Tromp, A., Wang, H., Hall, T.E., Mowry, B., Giacomotto, J.
- Source
- Full text @ Front. Physiol.
|
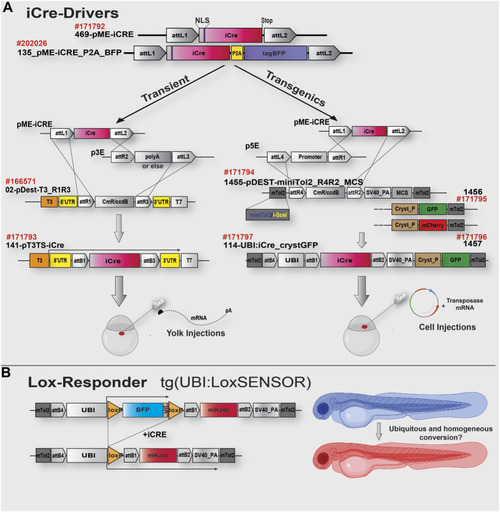
iCre toolkit and cloning strategy schematics. |
|
Viability of embryos and recombination efficiencies with iCre-mRNA, Cre-protein or transgenics. |
|
iCre-mRNA injections successfully trigger Lox-recombination but still with some visible partial conversions. |
|
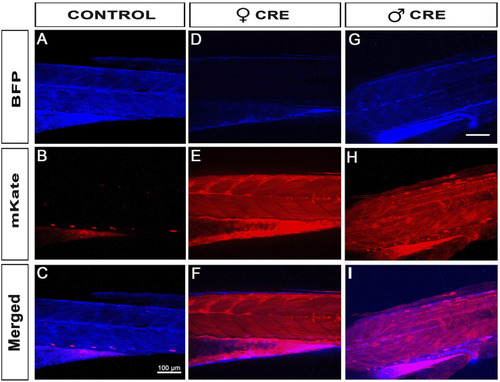
Female iCre-driver tg(UBI:iCre) triggers 100% recombination of Lox-Responder transgenes. |
|
Cre protein injections successfully trigger Lox-recombination. |