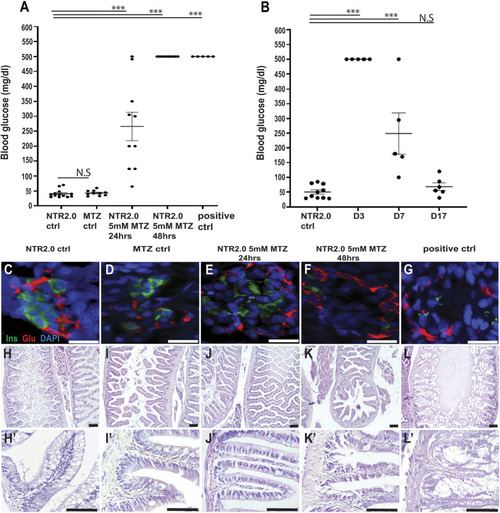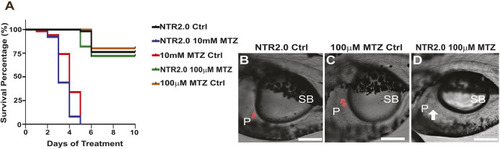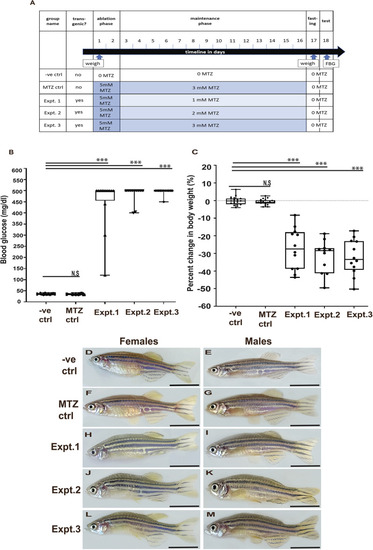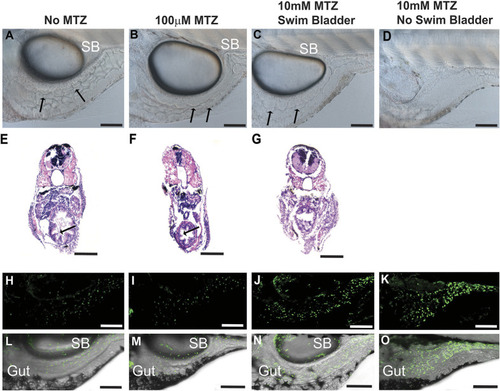
Deleterious effects of 10 mM MTZ on larval zebrafish. (A-O) Images taken from 5 dpf control (0 mM MTZ) larvae (A,E,H,L) or larvae treated from 3 to 5 dpf with either 100 µM MTZ (B,F,I,M) or 10 mM (C,D,G,J,K,N,O). (A,B) Images from the trunk region of casper larvae, showing that control (A) and 100 µM MTZ-treated (B) larvae have discernible gut lumens (complete with normal intestinal folds) as well as inflated swim bladders. (C,D) Surviving larvae treated with 10 mM display less defined gut lumen with few intestinal folds; some larvae have inflated swim bladders (C) and others have no inflated swim bladder (D) (20×, scale bars: 100 µm). (E-G) Transverse sections through the trunk stained with H&E to observe intestinal morphology confirmed a lack of intestinal folds in 10 mM MTZ-treated larvae with an inflated swim bladder (G) (20×, scale bars: 100 µm). (H-O) Images from 8xNFκB:eGFP 5dpf larvae indicate that 10 mM MTZ treatment leads to an increase in the number of cells undergoing NFκB signaling (J,K,N,O) (20×, scale bars: 100 µm). Black arrows point to intestinal folds. MTZ, metronidazole; SB, swim bladder.
|