- Title
-
A new microfluidic model to study dendritic remodeling and mitochondrial dynamics during axonal regeneration of adult zebrafish retinal neurons
- Authors
- Van Dyck, A., Masin, L., Bergmans, S., Schevenels, G., Beckers, A., Vanhollebeke, B., Moons, L.
- Source
- Full text @ Front. Mol. Neurosci.
|
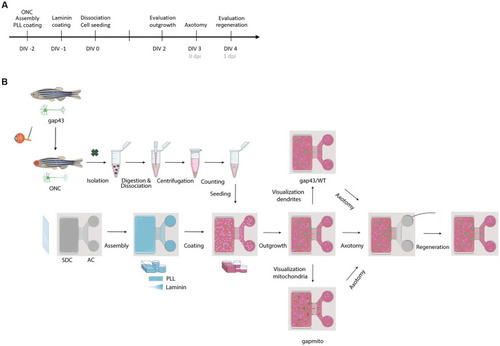
Schematic representation of the experimental setup. |
|
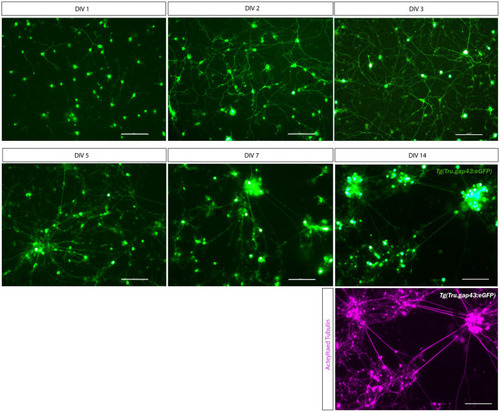
Long-term primary cell culture of adult zebrafish retinal neurons at DIV 1–14. Adult zebrafish retinal neurons can successfully be cultured using our newly established protocol. Live cell images taken at DIV 1–14 in a |
|
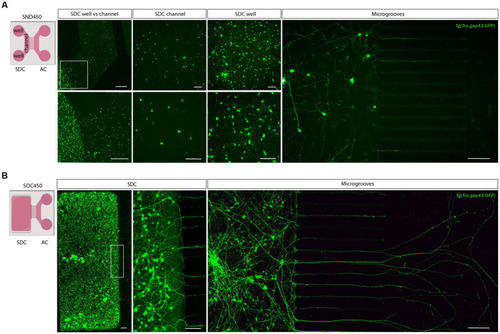
Adult zebrafish retinal cell culture in a microfluidic setup at DIV 3. |
|
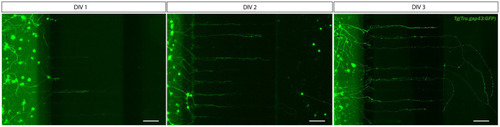
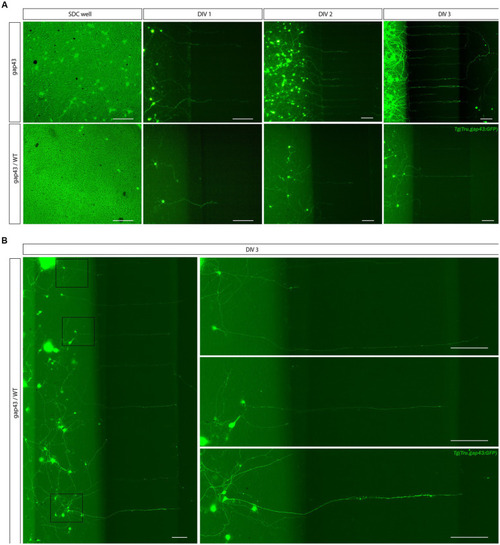
Outgrowth of adult zebrafish RGC axons at DIV 1–3 in a microfluidic setup. Adult zebrafish retinal neurons can successfully be cultured in an open (SOC450) MFD. Confocal live cell images indicate that adult |
|
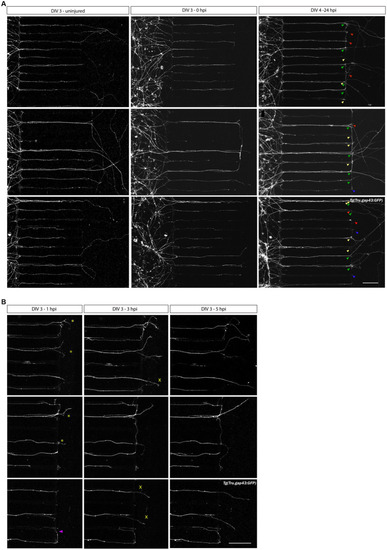
Axotomy and regeneration of adult zebrafish RGCs at DIV 3–4. Representative confocal live cell microscopy images taken before, upon and after injury in the same section of the AC, disclose that adult zebrafish RGCs regenerate spontaneously upon |
|
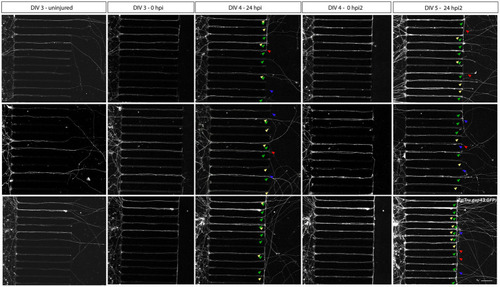
Axotomy and regeneration of repeatedly injured adult zebrafish RGCs at DIV 3–5. Adult zebrafish RGC axons that have successfully sprouted after a first |
|
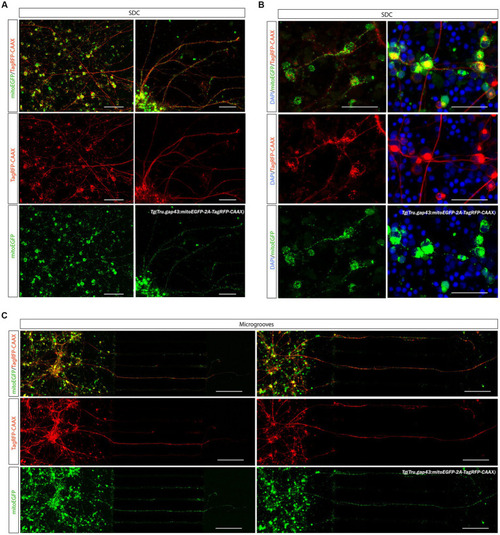
Creation of a sparsely labeled mixed culture to visualize individual adult zebrafish RGCs. To enable characterization of dendritic remodeling during axonal outgrowth, a sparsely labeled, mixed zebrafish retinal cell culture is created. |
|
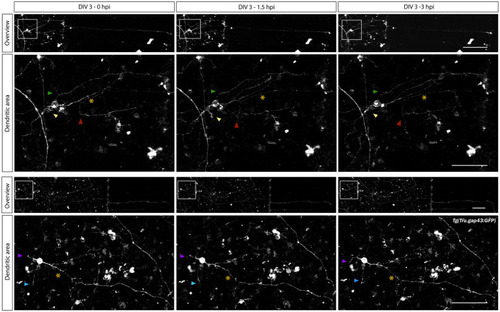
Characterization of changes in dendrite-like neurites during adult zebrafish RGC axonal regeneration. Time-lapse live imaging in sparesely labeled, mixed adult zebrafish retinal cultures seeded in a SOC450 MFD enables characterization of dendritic remodeling during axonal regeneration of adult zebrafish RGCs. Representative confocal still overview pictures of isolated gap43 RGCs (top panels) and detailed images of the dendritic area (bottom panels, with colored arrowheads indicating the same dendrite-like neurites over time and * indicating the axotomized axon) in a mixed |
|
Visualization of mitochondria in adult zebrafish RGCs in microfluidic cultures. The recently developed |
|
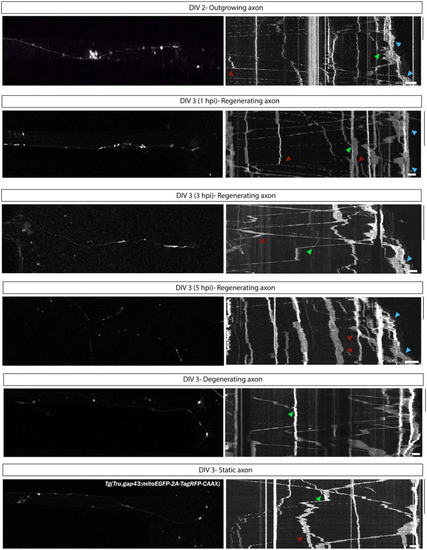
Characterization of mitochondrial motility during axonal outgrowth and regeneration of adult zebrafish RGCs. To characterize mitochondrial dynamics in outgrowing and regenerating RGCs, kymographs of time-lapse live movies, acquired in adult zebrafish retinal neurons cultured in an open compartment (SOC450) MFD, have been generated. Representative confocal still images from time-lapse live recordings in |