- Title
-
Circadian regulation of developmental synaptogenesis via the hypocretinergic system
- Authors
- Du, X.F., Li, F.N., Peng, X.L., Xu, B., Zhang, Y., Li, G., Liu, T., Li, Y., Wang, H., Yan, J., Du, J.L.
- Source
- Full text @ Nat. Commun.
|
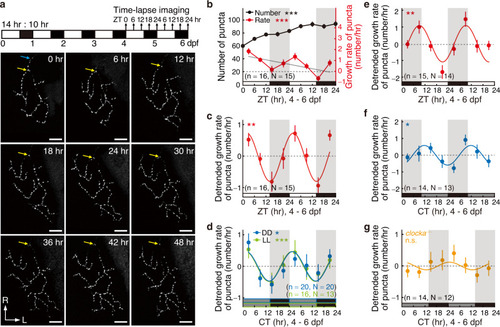
Circadian rhythm of retinotectal synaptogenesis during development in larval zebrafish in vivo. |
|
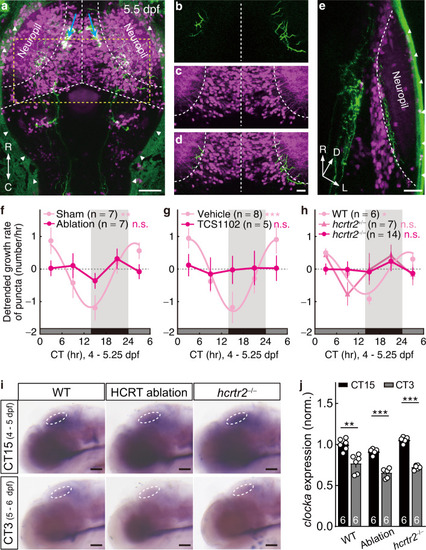
Hypocretinergic system is required for the circadian rhythm of synaptogenic rate. |
|
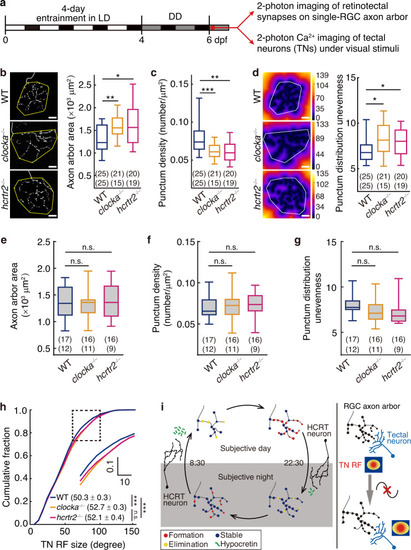
Circadian rhythm of synaptogenesis affects the arrangement of retinotectal synapses and the refinement of visual function. |