FIGURE SUMMARY
- Title
-
Stress resilience is established during development and is regulated by complement factors
- Authors
- Swaminathan, A., Gliksberg, M., Anbalagan, S., Wigoda, N., Levkowitz, G.
- Source
- Full text @ Cell Rep.
|
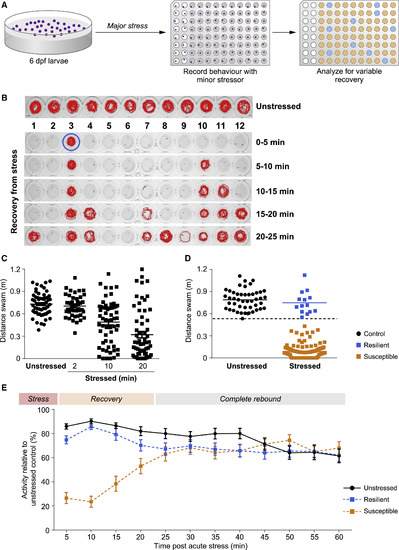
Figure 1Individual variability in stress rebound in zebrafish larvae (A) Scheme detailing the two-step resilience paradigm. Firstly, 6 dpf larvae were subjected to either osmotic or netting stress (major stressor) or left unstressed, and each larva was transferred into single wells of a 96-well plate. Then, the recovery of stressed (gray wells) and unstressed (white wells) larvae was monitored under a mild stressful challenge (dark novel environment) and subsequently analyzed. (B) Locomotive traces representing the spectrum of behaviors in the resilience paradigm. Unstressed larvae show explorative behavior, while larvae that underwent major stressful challenge prior to recording show different dynamics of recovery. Lanes 1–12 each represent snapshots of single larva recovering over time. (C and D) Dot plots representing the distance swam during the first 5 min of the recovery phase of larvae that were left unstressed or pre-subjected to a major stressor for increasing periods of time (C). Fast recoverers (resilient, blue) and slow recoverers (susceptible, yellow) individuals in (D) are separated by a dashed line, which represents a statistically calculated threshold. This threshold was determined by calculating the data point where the combined value of Fisher’s exact p and Cohen’s D comparing the two archetypes is most significant, indicating maximal difference (for details see STAR Methods). n = 47/144, unstressed/stressed. (E) Temporal analysis of recovery from a 20 min stressful challenge. The difference between the behavior of these groups is most evident in the variable recovery phase, following which the behavior of the three groups is comparable in the complete rebound phase. n = 30/group. Data are presented as mean ± SEM. PHENOTYPE:
|
|
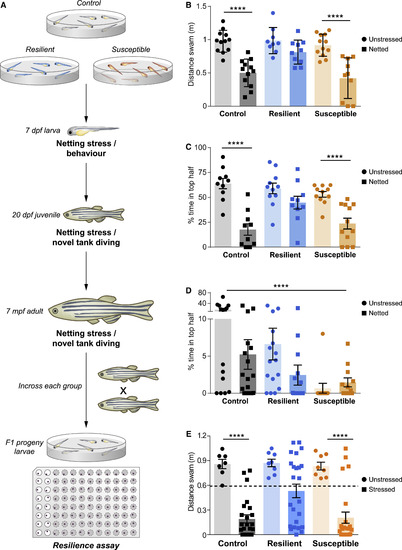
Figure 2Resilience and susceptibility are persistent and heritable traits (A) Scheme detailing the two-step resilience paradigm. Firstly, 6 dpf larvae were subjected to either osmotic or netting stress (major stressor) or left unstressed, and each larva was transferred into single wells of a 96-well plate. Then, the recovery of stressed (gray wells) and unstressed (white wells) larvae was monitored under a mild stressful challenge (dark novel environment) and subsequently analyzed. (B) Locomotive traces representing the spectrum of behaviors in the resilience paradigm. Unstressed larvae show explorative behavior, while larvae that underwent major stressful challenge prior to recording show different dynamics of recovery. Lanes 1–12 each represent snapshots of single larva recovering over time. (C and D) Dot plots representing the distance swam during the first 5 min of the recovery phase of larvae that were left unstressed or pre-subjected to a major stressor for increasing periods of time (C). Fast recoverers (resilient, blue) and slow recoverers (susceptible, yellow) individuals in (D) are separated by a dashed line, which represents a statistically calculated threshold. This threshold was determined by calculating the data point where the combined value of Fisher’s exact p and Cohen’s D comparing the two archetypes is most significant, indicating maximal difference (for details see STAR Methods). n = 47/144, unstressed/stressed. (E) Temporal analysis of recovery from a 20 min stressful challenge. The difference between the behavior of these groups is most evident in the variable recovery phase, following which the behavior of the three groups is comparable in the complete rebound phase. n = 30/group. Data are presented as mean ± SEM. |
|
Figure 3Resilient larvae show unique stress-responsive changes in the expression of neuropeptides and innate immune factors (A) Scheme detailing the two-step resilience paradigm. Firstly, 6 dpf larvae were subjected to either osmotic or netting stress (major stressor) or left unstressed, and each larva was transferred into single wells of a 96-well plate. Then, the recovery of stressed (gray wells) and unstressed (white wells) larvae was monitored under a mild stressful challenge (dark novel environment) and subsequently analyzed. (B) Locomotive traces representing the spectrum of behaviors in the resilience paradigm. Unstressed larvae show explorative behavior, while larvae that underwent major stressful challenge prior to recording show different dynamics of recovery. Lanes 1–12 each represent snapshots of single larva recovering over time. (C and D) Dot plots representing the distance swam during the first 5 min of the recovery phase of larvae that were left unstressed or pre-subjected to a major stressor for increasing periods of time (C). Fast recoverers (resilient, blue) and slow recoverers (susceptible, yellow) individuals in (D) are separated by a dashed line, which represents a statistically calculated threshold. This threshold was determined by calculating the data point where the combined value of Fisher’s exact p and Cohen’s D comparing the two archetypes is most significant, indicating maximal difference (for details see STAR Methods). n = 47/144, unstressed/stressed. (E) Temporal analysis of recovery from a 20 min stressful challenge. The difference between the behavior of these groups is most evident in the variable recovery phase, following which the behavior of the three groups is comparable in the complete rebound phase. n = 30/group. Data are presented as mean ± SEM. |
|
Figure 4Complement inhibition is a key determinant of early life stress resilience (A) Scheme detailing the two-step resilience paradigm. Firstly, 6 dpf larvae were subjected to either osmotic or netting stress (major stressor) or left unstressed, and each larva was transferred into single wells of a 96-well plate. Then, the recovery of stressed (gray wells) and unstressed (white wells) larvae was monitored under a mild stressful challenge (dark novel environment) and subsequently analyzed. (B) Locomotive traces representing the spectrum of behaviors in the resilience paradigm. Unstressed larvae show explorative behavior, while larvae that underwent major stressful challenge prior to recording show different dynamics of recovery. Lanes 1–12 each represent snapshots of single larva recovering over time. (C and D) Dot plots representing the distance swam during the first 5 min of the recovery phase of larvae that were left unstressed or pre-subjected to a major stressor for increasing periods of time (C). Fast recoverers (resilient, blue) and slow recoverers (susceptible, yellow) individuals in (D) are separated by a dashed line, which represents a statistically calculated threshold. This threshold was determined by calculating the data point where the combined value of Fisher’s exact p and Cohen’s D comparing the two archetypes is most significant, indicating maximal difference (for details see STAR Methods). n = 47/144, unstressed/stressed. (E) Temporal analysis of recovery from a 20 min stressful challenge. The difference between the behavior of these groups is most evident in the variable recovery phase, following which the behavior of the three groups is comparable in the complete rebound phase. n = 30/group. Data are presented as mean ± SEM. |
Acknowledgments
This image is the copyrighted work of the attributed author or publisher, and
ZFIN has permission only to display this image to its users.
Additional permissions should be obtained from the applicable author or publisher of the image.
Full text @ Cell Rep.