- Title
-
Next-Generation SINE Compound KPT-8602 Ameliorates Dystrophic Pathology in Zebrafish and Mouse Models of DMD
- Authors
- English, K.G., Reid, A.L., Samani, A., Coulis, G.J.F., Villalta, S.A., Walker, C.J., Tamir, S., Alexander, M.S.
- Source
- Full text @ Biomedicines

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. PHENOTYPE:
|
|
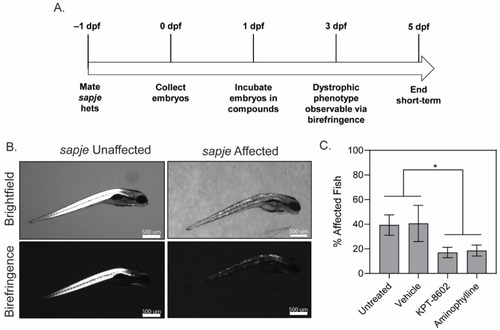
KPT−8602 improves dystrophic muscle pathology in zebrafish larvae. (A) Zebrafish drug dosing timeline; (B) Representative images of brightfield and birefringence for both unaffected (WT) and affected (sapje) zebrafish larvae. Scale bars represent 500 μm; (C) Graph summarizing the birefringence score (in percentage of affected/unaffected over total number of larvae assessed in the clutch) across untreated, vehicle-treated, KPT−8602-treated and aminophylline-treated (positive control) fish. KPT−8602 and aminophylline treatment significantly reduced the number of affected fish compared to untreated and vehicle-treated fish (data is represented as mean ± SEM, n = 60 per cohort, across 3 independent trials of n = 20 each; * p < 0.05; one-way ANOVA with Tukey’s HSD test). PHENOTYPE:
|
|
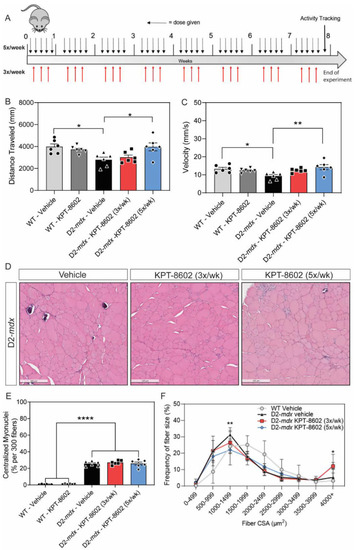
KPT−8602 treatment improves basal locomotion and histological markers in DMD mouse muscle. (A) Schematic of mouse experiments illustrating the timeline of dosage in each condition and activity tracking (B,C). Graphs summarizing total distance traveled (B) and average velocity (C) in an open field test. KPT−8602 treatment in D2-mdx mice significantly increased total distance travelled and average velocity compared to vehicle-treated D2-mdx mice (data is presented as mean ± SEM, n = 6; * p < 0.05, ** p < 0.01; one-way ANOVA with Tukey’s HSD test); (D) Representative images of tibialis anterior (TA) muscles stained with H&E across D2-mdx mice treated with either vehicle, 3x/week KPT−8602 or 5x/week KPT−8602. Scale bar = 200 µm; (E) KPT−8602 treatment did not reduce the number of centralized myonuclei, which is significantly increased in DMD pathology (data is presented as mean ± SEM, n = 6; **** p < 0.0001; one-way ANOVA with Tukey’s HSD test); (F) Fiber size frequency distribution curves of the cross-sectional area of myofibers revealed that KPT−8602 treatment decreased the number of small fibers and increased number of large fibers, indicative of a hypertrophy response compared to D2-mdx vehicle (data points are presented as mean ± SEM, n = 6; * p < 0.05, ** p < 0.01; one-way ANOVA with Tukey’s HSD test). |
|
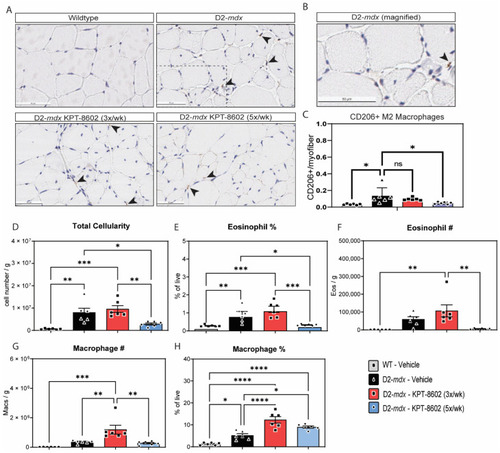
KPT−8602 treatment shifts D2-mdx muscles to a pro-regenerative, M2 macrophage profile. (A) Immunostaining of CD206-positive TA muscles from WT-vehicle, D2-mdx vehicle, D2-mdx KPT−8602 3x/week, and D2-mdx 5x/week experimental mouse cohorts. Arrows indicate CD206 stained macrophages. Boxed area magnified in B. Scale bar = 50 µm; (B) Magnified inset of a CD206+ macrophage from the D2-mdx sample; (C) Quantification of the total number of CD206+ M2 macrophages normalized to the total number of myofibers per area quantified to a minimum of 500 myofibers. All data is presented as mean ± SEM, n = 6; * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001; one-way ANOVA with Tukey’s HSD test); (D) Total cellularity measured in quadricep muscle cell preparations from the 4 experimental cohorts: WT vehicle (grey), D2-mdx vehicle (black), D2-mdx 3x/week (red); D2-mdx 5x/week (blue); (E,F) graphs showing the percentage of eosinophils and the total eosinophil number/gram muscle wet weight; (G,H) Graphs showing the percentage of macrophages and the total macrophage number/gram muscle wet weight. |
|
KPT−8602 reduces osteopontin serum levels in D2-mdx mice. Osteopontin (OPN) in circulating serum levels was assessed by ELISA assay. KPT−8602 (5x/week) treatment significantly reduced osteopontin levels compared to vehicle-treated D2-mdx, demonstrating levels comparable to WT mice (data is presented as mean ± SEM, n = 4–5 mice per cohort; * p < 0.05, ** p < 0.01; one-way ANOVA with Tukey’s HSD test). |