- Title
-
A robot-assisted acoustofluidic end effector
- Authors
- Durrer, J., Agrawal, P., Ozgul, A., Neuhauss, S.C.F., Nama, N., Ahmed, D.
- Source
- Full text @ Nat. Commun.
|
Experimental set-up and working principle of the robot-assisted acoustofluidic end effector (RAEE) device.
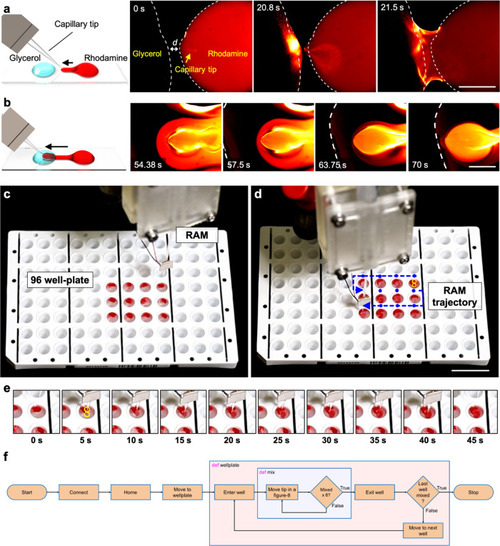
a The RAEE device is comprised of a glass capillary and piezo-electronic transducer connected to a five-axis robotic arm. The whole set-up was mounted on an inverted microscope using a capillary holder. The inset illustrates the overall experimental set-up. b An enlarged view of the RAEE device when submerged in liquid with a focus on streaming profiles, especially the out-of-plane streaming. c The acoustofluidic device generated two distinct flow profiles: (i) circular-flow fields along the shaft of the capillary, and (ii) frequency-dependent 3D streaming at the tip of the glass capillary. d We have applied the RAEE to execute pumping, selective trapping, and viscous mixing. |
|
|
|
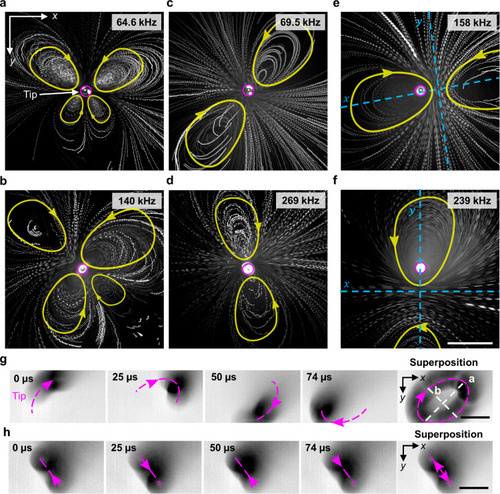
The acoustofluidic device produced steady 3D vortex flow patterns comprised of counter-rotating vortices (The pink circle represents the capillary tip position and yellow geometry represents the direction and path of motion of particles.) (see Supplementary Movie |
|
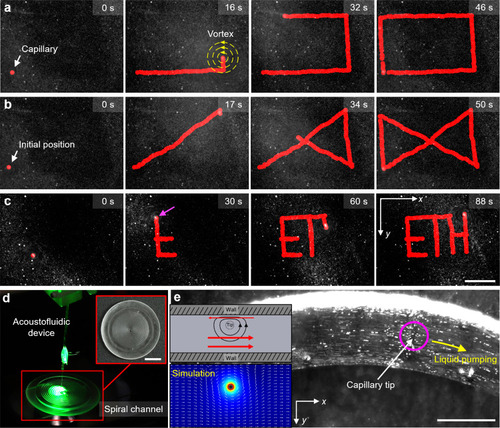
Image sequences depicting the robot-assisted acoustofluidic device executing automated patterns (here the red marking represents the path of the capillary tip and yellow concentric circles represents the vortex generated) (see Supplementary Movie |
|
|
|
|