- Title
-
Dichloroacetate improves mitochondrial function, physiology, and morphology in FBXL4 disease models
- Authors
- Lavorato, M., Nakamaru-Ogiso, E., Mathew, N.D., Herman, E., Shah, N.K., Haroon, S., Xiao, R., Seiler, C., Falk, M.J.
- Source
- Full text @ JCI Insight
|
Protein alignment and evolutionary conservation of FBXL proteins in Homo sapiens, D. rerio, and C. elegans. Protein alignment of H. sapiens, D. rerio, and C. elegans (see Supplemental Figure 1 for alignment with other species). The locations of mutations in the zebrafish and C. elegans mutant strains studied are shown: c.813T>A, p.Y271X in zebrafish and g.410_1116del, p.Phe105_Lys308del in C. elegans. Protein alignment of conserved F-box–like domain among H. sapiens, D. rerio, and C. elegans is shown. Percentage homology is shown in gray, and white and black indicate the lowest and highest levels of similarity, respectively. |
|
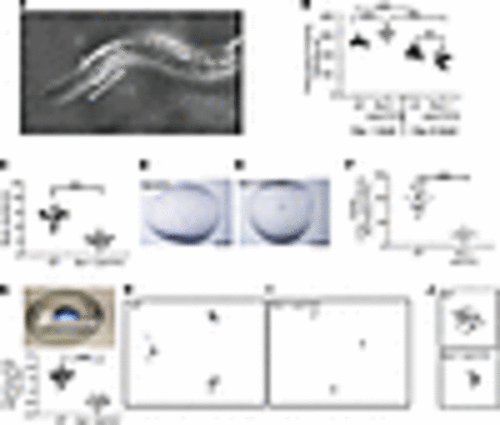
fbxl-1(ok3741) worms had abnormal development, growth, and fecundity. (A) At 64 hours after eggs were laid, 48% of fbxl-1(ok3741) worms had reached L3 larval development stage and 52% had reached the L4 larval stage. By contrast, 39% of N2 WT worms were at L4 larval development stage and 61% had reached the young adult stage, with no worms seen at the earlier larval stages. n = 168 N2 WT worms; n = 152 fbxl-1(ok3741) worms; 3 biological replicates. (B and C) fbxl-1(ok3741) worm average body length was significantly decreased, and averaged body width was significantly increased, compared with WT worms. n = 54 WT; n = 46 fbxl-1(ok3741). (D) fbxl-1(ok3741) brood size was 58% smaller than that of N2 WT worms. n = 13 N2 WT adult worms; n = 14 fbxl-1(ok3741) adult worms; 3 biological replicates. (E) fbxl-1(ok3741) egg-hatching rate was 21% lower than that in N2 WT worms. n = 136 each strain; 3 biological replicates. (F and G) Images of WT and fbxl-1(ok3741) gravid worms at day 2 of the adult stage. fbxl-1(ok3741) gravid worms have disarranged eggs with differing polarity (G, arrowheads) as compared with WT gravid worms, in which eggs are organized in 1 or 2 layers with similar polarity (F, arrowheads). Data are shown as the mean ± SD. Statistical analyses were performed by Student’s t test. *P < 0.05, **P < 0.01, ***P < 0.001. Scale bar: 400 μm. |
|
fbxl-1(ok3741) worms had impaired neuromotor function. (A) Pharyngeal pump rate experimental overview (Supplemental Video 1). WT C. elegans animal observed under dissecting microscope using oblique illumination; the grinder appears as a white dot (arrow) in terminal bulb. (B) Pharyngeal pumping rates of fbxl-1(ok3741) and WT worms at 1- and 6-day adult stage (n = 20 per strain at 1-day adult stage; n = 25 per strain at 6-day adult stage). Bonferroni’s correction method was applied to account for multiple comparisons, and significant findings still held. (C) fbxl-1(ok3741) and WT body bend rate on agar plates (Supplemental Videos 2 and 3). n = 25 worms per strain; 5 biological replicates performed. (D–F) Worm swimming activity analysis in liquid media. (D and E) Five worms were transferred to each drop of liquid media (scale bar: 1 mm) on a glass slide. Activity of all 5 worms in aggregate was recorded, and pixel change was analyzed using ZebraLab. (F) fbxl-1(ok3741) worm swimming activity was significantly lower than WT worms (Supplemental Videos 4 and 5). Each dot indicates the total activity of 5 worms in a single technical replicate. n = 12 for WT; n = 11 for fbxl-1(ok3741); with a total of 60 WT and 55 fbxl-1(ok3741) worms analyzed across 4 biological replicates. (G–J) Thrashing activity assay. (G) Each fbxl-1(ok3741) and WT worm was transferred to 5 μL liquid media for individual worm activity analysis by wrMTrck plugin (ImageJ). The graph shows the BBPS, which were significantly decreased in fbxl-1(ok3741) worms as compared with WT worms. Each dot indicates activity of an individual worm. n = 36 each strain. (H and I) Representative traces of worm movements detected by wrMtrck analysis. fbxl-1(ok3741) worms showed less activity and uncoordinated movements than WT worms. (J) Higher-magnification images of representative individual fbxl-1(ok3741) and WT worm traces. Data are shown as the mean ± SD. Significance was determined using unpaired Student’s t test. *P < 0.05, **P < 0.01, ***P < 0.001. |
|
DCA treatment rescued egg-laying behavior, pharyngeal pumping function, and mitochondrial function in fbxl-1(ok3741) worms. (A) Visualization of worm progeny using semiautomated Wormscan method, showing reduced density of fbxl-1(ok3741) worms as compared with WT worms in control media, and recovered density of signal in the well with DCA-treated fbxl-1(ok3741) worms. (B) Difference image score obtained with WormScan method and normalized using percentage of control (POC); fbxl-1(ok3741) worms (n = 38) and WT worms at baseline (n = 35); n = 57 for 25 mM DCA treated bxl-1(ok3741) and n = 31 for DCA-treated WT worms; (mean ± SEM). (C) Pharyngeal pump rate in untreated and DCA-treated (25 mM) 1-day adult fbxl-1(ok3741) and N2 WT worms. n = 25 worms/strain in control media; n = 20 worms/strain in DCA media; 3 biological replicates. (D) Untreated and DCA-treated worms’ swim activity analyzed by ZebraLab; n = 12 DCA-treated worms; n = 11 untreated worms; (mean ± SD). (E–H) RC enzyme activity of CS, complex I (CI), CII, and CIV was quantified in untreated and DCA-treated WT and fbxl- 1(ok3741) synchronized day-1 adult worm populations. All rates are expressed as percentage of normalized WT control. n = 3 per condition; (mean ± SEM). One-tailed t test performed for statistical analysis. (I) ATP levels in whole worms were unchanged in fbxl-1(ok3741) worms relative to WT worms in untreated and DCA-treated conditions (mean ± SEM). n = 5 each strain. (J) Whole worm lactate evaluated in fbxl-1(ok3741) worms relative to WT worms (mean ± SEM). n = 5 each strain. Significance was determined using unpaired Student’s t test. *P < 0.05, **P < 0.01, ***P < 0.001. Bonferroni’s correction method was applied to account for multiple comparisons, and significant findings still held. |
|
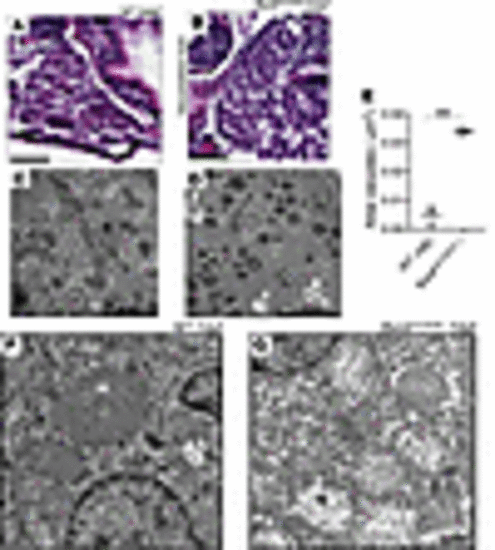
Biochemical analysis, histopathology, and ultrastructural investigation of homozygous fbxl4sa12470 zebrafish liver. (A) Liver histology in AB (WT) zebrafish larvae at 6 dpf. Scale bar: 25 μm. (B) Vacuolated liver in homozygous fbxl4sa12470 zebrafish larvae at 6 dpf. 100% of larvae (n = 25) showed vacuolated liver, while no WT larvae (n = 12) showed the disease phenotype. Scale bar: 25 μm. (C) Ultrastructure of hepatocytes in 7 WT dpf zebrafish larvae showing normal ultrastructure. Scale bar: 10 μm. (D) Ultrastructure of hepatocytes in mutant 7 dpf zebrafish larvae showing increased rate of lipid droplets (L) and autophagic vacuoles (white arrows). Scale bar: 10 μm. (E) Total area of vacuoles occupying liver areas estimated in μm2 in mutant versus WT larvae at 7 dpf. Total area of liver analyzed: 1,978 μm2 (WT) and 2,741 μm2 (fbxl4sa12470). **P < 0.01. Significance was determined using unpaired t test. n = 3 each fish line. (F) Mitochondrial ultrastructure (M) in hepatocytes of 7 dpf WT zebrafish larvae. (G) Loss of normal matrix electron density and mitochondrial cristae damage (M) was observed in hepatocytes of 7 dpf fbxl4sa12470–/– larvae. Scale bar: 1 μm (F and G). |
|
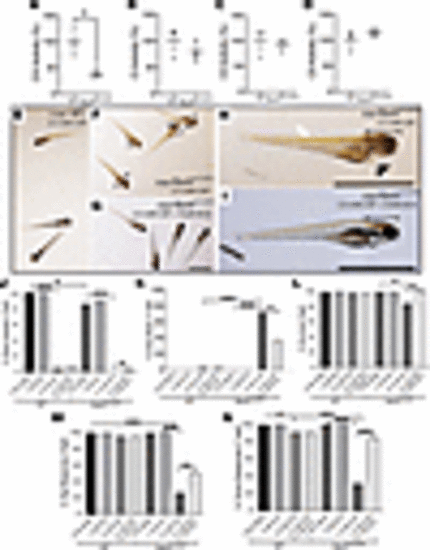
DCA rescued the gray brain phenotype, survival, and integrated neurologic and/or muscular function in CAP-stressed fbxl4sa12470 larval zebrafish. (A–D) RC and CS enzyme activity detected in 7 dpf larvae (mean ± SEM). n = 3 each condition. (E–I) Representative images of 6 dpf age-matched fbxl4sa12470 and AB WT larvae at 6 dpf after 4 days (starting from 2 dpf) of incubation with 2.5 mM CAP alone or with DCA cotreatment. Morphological defects were obvious at 6 dpf, showing higher sensitivity of (F and H) fbxl4sa12470 larvae to 2.5 mM CAP compared with (E) AB WT larvae: gray brain phenotype (black arrows in F and bracket in H, Supplemental Figure 3), heart edema (generally not observed in WT, arrowheads in F and H), and overall body degeneration and slight bent tail (white arrow in H). (G and I) Coexposure of stressed fbxl4sa12470 larvae with 5 mM DCA improved the gray brain phenotype (clear brain in I indicated by a bracket; Supplemental Figure 3) but did not rescue the delay in swim bladder formation in either AB WT or fbxl4sa12470 larvae. Scale bar: 1 mm. (J–N) 2.5 mM CAP significantly affected development (percentage of swim bladder), survival, and neuromuscular response (percentage of tap response and touch response), and caused brain death in 7 dpf mutant larvae (Supplemental Table 2, A and B). (J and K and Supplemental Videos 6 and 7) 5 mM DCA significantly rescued the gray brain phenotype and survival and improved neuromuscular response. *P < 0.05, **P < 0.01, ***P < 0.001, Cochran-Mantel-Haenszel and χ2 test performed (Supplemental Table 2). Bar graphs are representative of the statistical analysis shown in the Supplemental Table 2, where all data details are shown. PHENOTYPE:
|
|
FBXL4–/– human fibroblasts had reduced CS activity and abnormal mitochondrial structure that was improved with DCA treatment. (A) CS activity decreased in patient fibroblasts from participants 1 (n = 3) and 2 (n = 5) as compared with control fibroblasts (n = 3). (B–D) RC enzyme activity was not significantly changed in patient fibroblasts compared with control fibroblasts. (E) Extracellular lactate levels were significantly decreased in patient fibroblasts compared with control cells in control media. DCA treatment did not affect extracellular lactate levels in patient cells. n = 12 each condition. (F) Intracellular lactate level evaluation in untreated and DCA-treated (20 mM) fibroblasts from participant 1 and controls. n = 12 each condition. (G) CS activity after treatment of fibroblasts with 20 mM DCA (participants 1 and 2). CS activity level in treated fibroblasts was normalized for percentage in control media. Data are shown as the mean ± SEM. Significance was determined using unpaired Student’s t test. *P < 0.05, ***P < 0.001. Bonferroni’s correction method was applied for A and E–G to account for multiple comparisons, and significant findings still held. (H and I) Low-magnification images (original magnification, ×20) of FBXL4–/– fibroblasts from participant 1 after exposure to metabolic stressor and cotreatment with 20 mM DCA for 48 hours. Mitochondria were stained with MitoTracker green (Thermofisher Scientific). Scale bar: 200 μm. (J and K) High-magnification images of mitochondrial morphology in FBXL4–/– fibroblasts from participant 1 after exposure to metabolic stressor and cotreatment with 20 mM DCA for 48 hours (see representative fibroblast image from participant 2 in Supplemental Figure 5). Mitochondria (in green) were disarranged and fragmented when incubated with stressor. More elongated mitochondria were visible after coincubation with 20 mM DCA. Scale bar: 25 μm. (L and M) EM images of mitochondria in FBXL4–/– fibroblasts from participant 1, showing loss of matrix electron density and loss of mitochondrial cristae after incubation in metabolic stressor for 48 hours and rescue of mitochondrial ultrastructure, with defined cristae and normal matrix electron density after cotreatment with 20 mM DCA. Scale bar: 500 nm. |