- Title
-
Myoneurin regulates BMP signaling by competing with Ppm1a for Smad binding
- Authors
- Yang, S., Ning, G., Hou, Y., Cao, Y., Xu, J., Wu, J., Zhang, T., Wang, Q.
- Source
- Full text @ iScience
|
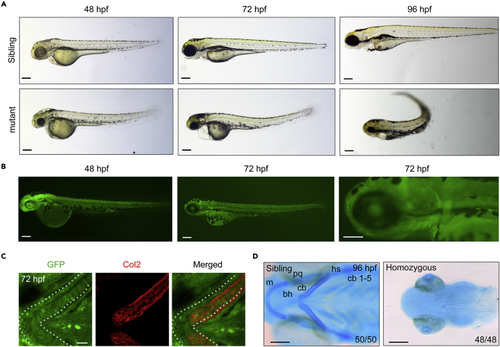
The GFP expression and developmental defects in T054 embryos (A) Morphology of T054 mutants at indicated stages. Note that mutant embryos had a darker head, rough skin, reduced size of head and eyes, severe craniofacial jaw malformations, and curly posterior trunk with stage-dependent degrees. Scale bars, 200 μm. (B) Fluorescent images of T054 embryos. The pictured embryos were heterozygotes. Scale bars, 200 μm. (C) Immunostaining of chondrocytes by anti-Col2 and GFP antibody. T054 heterozygous embryos at 72 hpf were co-immunostained with anti-Col2 (red) and anti-GFP (green) antibodies. Scale bar, 20 μm. (D) Craniofacial cartilages stained with Alcian blue. Note that almost no cartilages could be seen in the T054 mutant embryos. The cartilages were positioned with anterior to the left, and ceratobranchial (cb), ceratohyal (ch), Meckel’s cartilage (m), palatoquadrate (pq), and hyosymplectic (hs) cartilages were shown. Scale bars, 200 μm. EXPRESSION / LABELING:
|
|
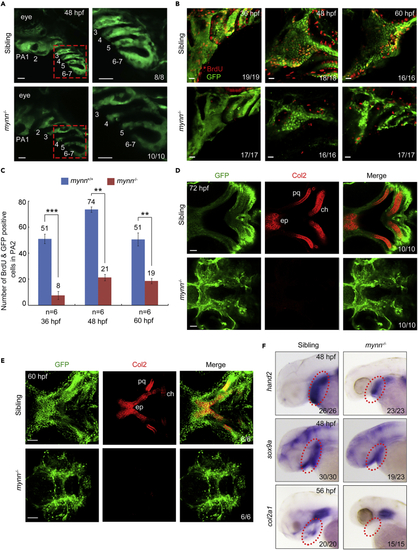
mynn was trapped in the T054 line (A) Genomic structure of the mynn locus and putative transcripts. Exons are numbered. The binding positions and directions of the primers used in (B) are indicated. Note that in the homozygous embryos, the transcripts of mynn only contain the first two exons at the upstream of the translational initiation site. (B) Genotyping of individual embryos by PCR. Embryos collected from mynn heterozygote intercrosses were separated based on GFP intensity. Three primers, p1, p2, and p3, were used together for PCR. The upper (a) and lower (b) bands represented the wild-type and recombinant allele, respectively. M, molecular marker; P, positive control; N, negative control with no template. (C) Expression pattern of mynn in wild-type embryos at indicated stages detected by in situ hybridization. The transcripts are overt in the pharyngeal region at 48 and 72 hpf as indicated by red arrowheads. (D) Expression pattern of mynn in embryos from different genotypes. Note that the mynn expression in mynn−/− embryos was almost disappeared. (E) RT-PCR analysis of mynn transcripts in wild-type and mutant embryos at 24 hpf. β-actin was used as an internal control. The data were presented as mean ± SD from three independent biological repeats. Total RNA of each group was extracted from a pool of 30 embryos. ∗∗, p < 0.01 (by Student’s t test). (F) Overexpression of mynn rescued the mutant phenotype. mynn heterozygote intercrosses were injected with 200 pg mynn mRNA at the one-cell stage. The homozygous mutants were sorted out at 24 hpf by their EGFP intensity, and their morphology was observed at 72 hpf. The ratio of embryos with presented morphology was indicated. Scale bars, 200 μm. (G) Head skeletons stained with Alcian blue. Note that cartilages of mynn−/− mutants were missed and the missing cartilages could be rescued by injection of 200 pg mynn mRNA into mynn−/− embryos at the one-cell stage or specific overexpression of mynn in sox10+ NCCs. Scale bars, 200 μm. EXPRESSION / LABELING:
|
|
Reduced cell proliferation and differentiation of CNCCs in mynn−/− embryos (A) Live imaging of the pharyngeal region of mynn−/− embryos in Tg(fli1:EGFP) background. The pharyngeal arches were numbered. The boxed areas in the left pannel were enlarged in the right panels. PA: pharyngeal arch. Scale bars, 20 μm. (B and C) BrdU incorporation experiments showed reduced proliferating CNCCs in mynn−/− mutants. BrdU-treated mynn−/− mutants with fli1:EGFP expression were harvested at indicated stages and stained with anti-BrdU (red) and anti-GFP (green) antibodies. The pharyngeal regions were observed by confocal microscopy (B). Scale bars, 20 μm. The number of BrdU+ and GFP+ cells in the second pharyngeal arch was calculated from six embryos (C). Error bars indicate ±S.D. ∗∗, p < 0.01; ∗∗∗, p < 0.001 (by Student’s t test). (D and E) Detection of Col2 proteins in the pharyngeal arches. Wild-type and mynn−/− embryos in Tg(fli1:EGFP) background at 60 (E) or 72 (D) hpf were co-immunostained with anti-GFP (green) and anti-Col2 (red) antibodies. The palatoquadrate (pq), ethmoid plate (ep), and ceratohyal (ch) cartilages were shown with anterior to the left. Scale bars, in panel D, 20 μm; in panel E, 50 μm. (F) Expression pattern of hand2, sox9a, and col2a1 in mynn−/− embryos and their siblings at indicated stages detected by in situ hybridization. EXPRESSION / LABELING:
PHENOTYPE:
|
|
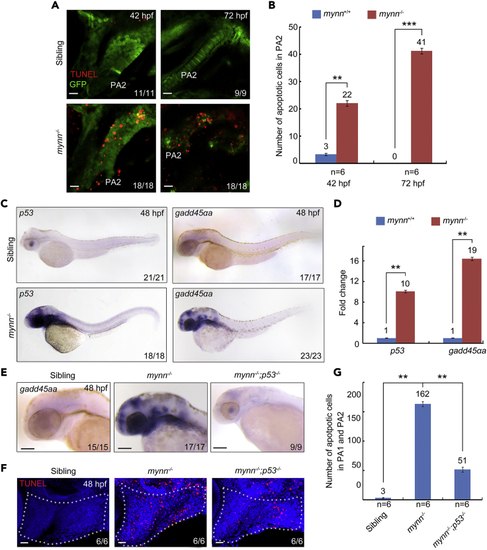
mynn mutants exhibit cell death and upregulated p53 signaling (A and B) Detection of apoptotic cells in the pharyngeal regions. mynn-deficient Tg(fli1:EGFP) embryos and their siblings were collected at indicated stages for TUNEL assay (A). PA, pharyngeal arch. Scale bars, 10 μm. The number of TUNEL+ and GFP+ cells in PA2 was calculated from six embryos (B). Error bars indicate ±S.D. ∗∗, p < 0.01; ∗∗∗, p < 0.001 (by Student’s t-test). (C) Expression pattern of p53 and gadd45αa in mynn−/− embryos at indicated stages. (D) Real-time PCR analysis of the relative expression level of p53 and gadd45αa in wild-type and mynn−/− embryos. β-actin was used as an internal control. Embryos were pre-sorted as mentioned above. The data were presented as mean ± SD from three independent biological repeats. Total RNA of each group was extracted from a pool of 30 embryos. ∗∗, p < 0.01 (by Student’s t-test). (E) Expression analysis of gadd45αa by in situ hybridization in mynn−/− mutants and mynn−/−;p53−/− embryos. (F and G) The apoptotic cells in the pharyngeal region were clearly decreased in mynn−/−;p53−/− embryos. Indicated embryos were collected at 48 hpf, and then subjected to TUNNEL assays. Representative images were shown in (F). The white dotted lines outline the pharyngeal regions. Scale bars, 20 μm. The number of TUNEL-positive cells in the pharyngeal regions was showed in (G). Error bars indicate ±S.D. ∗∗, p < 0.01 (by Student’s t-test). EXPRESSION / LABELING:
PHENOTYPE:
|
|
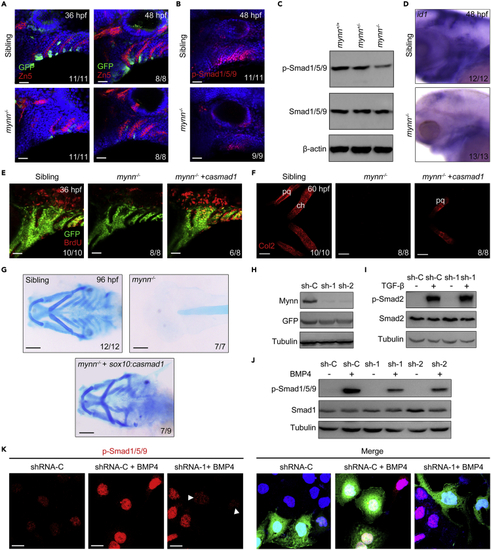
Mynn negatively regulates BMP signaling (A) Expression of dGFP reporter in the pharyngeal region. Mynn−/− mutants and their siblings in Tg(BRE:dGFP) background were stained with the indicated antibodies. Nuclei were counterstained with DAPI (blue). Lateral views, anterior to the left. Scale bars, 20 μm. (B and C) Expression of p-Smad1/5/9 was dramatically reduced in mynn−/− mutants. mynn−/− embryos and their siblings were harvested at 48 hpf, and then stained with anti-p-Smad1/5/9 antibodies (B). Scale bars, 20 μm. The levels of p-Smad1/5/9 in cell lysis of head region were further analyzed by Western blots (C). (D) Expression levels of BMP target gene id1 were detected by in situ hybridization. Embryos were lateral views with anterior to the left. (E) Reactivation of BMP signaling can restore the proliferation of CNCCs in mynn mutant embryos. mynn−/− embryos in Tg(fli1:EGFP) background were injected with or without 10 pg mRNA encoding constitutively active Smad1 protein (caSmad1) at the one-cell stage, and incubated with BrdU at 34 hpf, then harvested at 36 hpf for staining with anti-BrdU (red) and anti-GFP (green) antibodies. Scale bars, 20 μm. (F) Reactivation of BMP signaling can partially restore the differentiation of CNCCs in mynn−/− mutants. mynn−/− embryos were injected with or without 10 pg casmad1 mRNA at the one-cell stage and then immunostained with anti-Col2 antibody at 72 hpf. pq, palatoquadrate; ch, ceratohyal. Scale bars, 20 μm. (G) Alcian blue staining on embryos from different genotypes. Note that the missing cartilages in mynn−/− mutants could be rescued by specific overexpression of casmad1 in sox10+ NCCs. Scale bars, 200 μm. (H) The effectiveness of Mynn shRNAs. HEK293T cells were transfected with the indicated shRNA plasmids and harvested 48 h after transfection for Western blot analyses. (I and J) HEK293 cells transfected with indicated shRNA plasmids were treated with TGF-β1 (5 ng/mL) for 2 h (I) or BMP4 (20 ng/mL) for 4 h (J), and then collected for Western blots with the indicated antibodies. (K) Hep3B cells transfected with plasmids expressing indicated shRNAs and GFP proteins were treated with or without BMP4 for 4 h. The expression levels of p-Smad1/5/9 were determined with immunostaining. As indicated with white arrowheads, the expression of p-Smad1/5/9 was obviously decreased in the cells expressing Mynn shRNAs. Scale bars, 20 μm. PHENOTYPE:
|
|
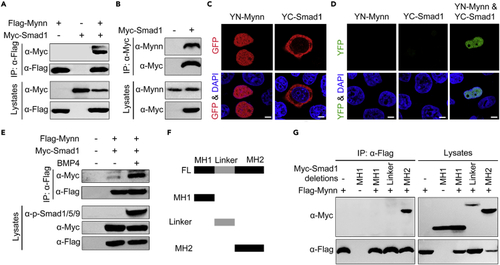
Mynn associates with Smad1 (A) Flag-tagged Mynn interacts with overexpressed Smad1. HEK293T cells were transfected with plasmids expressing Flag-Mynn and Myc-Smad1, and then harvested for immunoprecipitation with anti-Flag antibody. (B) Overexpressed Smad1 interacts with endogenous Mynn. HEK293T cells transfected with Myc-tagged Smad1 were subjected to immunoprecipitation with anti-Myc antibody. (C and D) Detection of Mynn-Smad1 interaction in living cells by BiFC assays. YN-Mynn and YC-Smad1 were transfected alone or simultaneously into HeLa cells. The expression of YN-Mynn or YC-Smad1 was detected by immunostaining with anti-GFP antibody (C). The reconstituted YFP fluorescence was detected by confocal microscopy at 488 nm (D). Scale bars: 10 μm. (E) BMP4 treatment enhanced the interaction between Mynn and Smad1. HEK293T cells transfected with Myc-tagged Smad1 and Flag-tagged Mynn, and then treated with or without BMP4 for 4 h before being harvested for immunoprecipitation. (F) Schematic representation of Smad1 truncations. (G) Mynn binds to the MH2 domain of Smad1. |
|
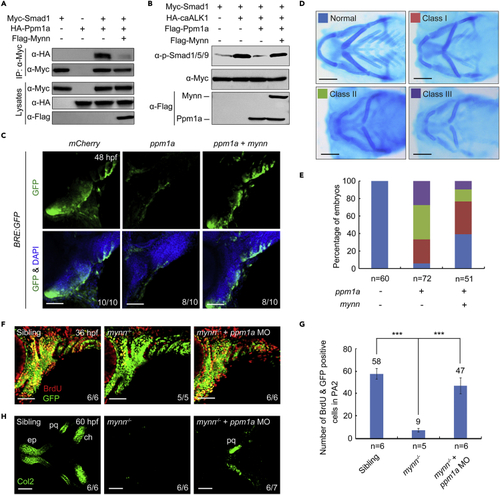
Mynn promotes BMP signaling through competing with Ppm1a for Smad binding (A) Presence of Mynn attenuates the interaction of Ppm1a and Smad1. HEK293T cells were co-transfected with Myc-Smad1 and HA-Ppm1a together with or without Flag-Mynn, and then harvested for immunoprecipitation. (B) Mynn weakens the Ppm1a-mediated dephosphorylation of p-Smad1/5/9. HEK293T cells were transfected with the indicated constructs. CaALK1 is a constitutively active form of BMP type 1 receptor ALK1 (Q233D). The expression levels of p-Smad1/5/9 and total Smad1 were detected by Western blots with appropriate antibodies. (C) Overexpression of mynn can restore the decrease of BMP signal activity caused by excessive Ppm1a. Tg(BRE:dGFP) embryos were injected with indicated mRNAs at the one-cell stage and subjected to immunostaining for GFP (green) at 48 hpf. Scale bars, 50 μm. Injection dosages: mCherry mRNA, 400 pg; ppm1a mRNA, 400 pg; mynn mRNA, 300 pg. (D and E) Ppm1a-induced pharyngeal cartilage defects were compromised by overexpression of mynn. Wild-type embryos were injected with 400 pg ppm1a mRNA together with or without 300 pg mynn mRNA at the one-cell stage, and stained at 96 hpf for cartilage with Alcian blue. Representative pictures of different classes of defects in pharyngeal cartilages were shown in (D). The percentage of embryos with different degrees of cartilage defects was calculated (E). Scale bars, 200 μm. (F and G) Knockdown of ppm1a could restore the CNCC proliferation defects in mynn−/− mutants. mynn−/− mutant embryos were injected with 5 ng ppm1a MO at the one-cell stage and incorporated with BrdU at 34 hpf, and then harvested at 36 hpf for immunostaining with anti-BrdU (red) and anti-GFP (green) antibodies (F). Scale bars, 50 μm. The number of BrdU and GFP double positive cells in the second pharyngeal arch was calculated (G). Error bars indicate ±S.D. Student’s t-test, ∗∗∗, p < 0.001. (H) The differentiation defects of CNCCs in mynn mutant embryos were partially recovered by ppm1a knockdown. mynn−/− embryos were injected with 5 ng ppm1a MO at the one-cell stage and harvested at 60 hpf for immunostaining with anti-Col2 (green) antibody. pq, palatoquadrate; ep, ethmoid plate; ch, ceratohyal. Scale bars, 50 μm. |

Unillustrated author statements PHENOTYPE:
|