- Title
-
Profilin 2 and Endothelial Exosomal Profilin 2 Promote Angiogenesis and Myocardial Infarction Repair in Mice
- Authors
- Li, Z., Huo, X., Chen, K., Yang, F., Tan, W., Zhang, Q., Yu, H., Li, C., Zhou, D., Chen, H., Zhao, B., Wang, Y., Chen, Z., Du, X.
- Source
- Full text @ Front Cardiovasc Med
|
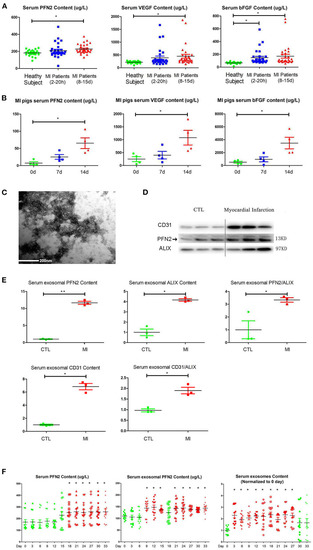
Increased PFN2 and exosomal PFN2 levels in the serum of patients with MI and in animal models. |
|
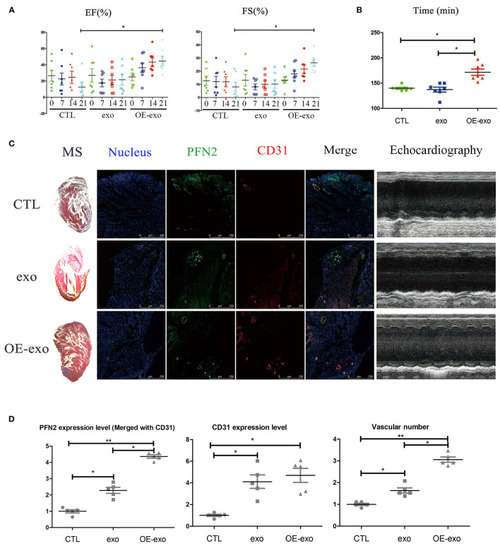
Exosomal PFN2 repairs infarction in mouse with MI and restores cardiac function. |
|
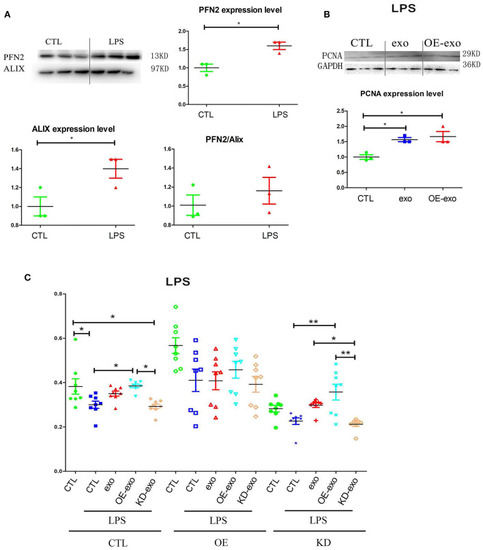
PFN2 protects ECs after LPS treatment. |
|
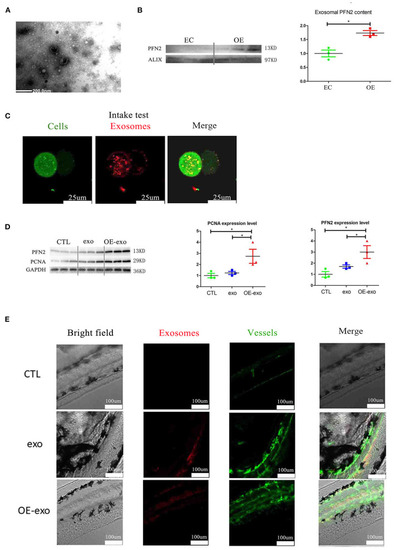
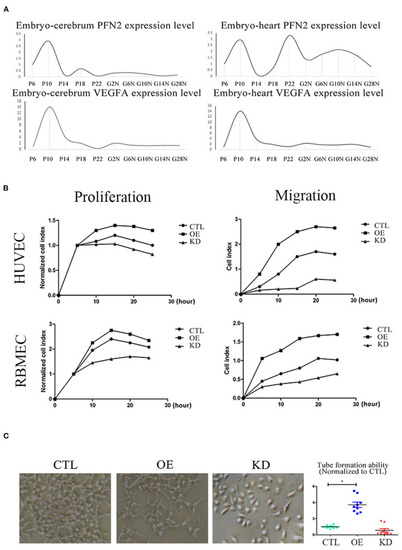
Exosomal PFN2 enhances the proliferation ability of ECs and OE-exo treated zebrafish showed significantly increased vessel number than exo and control. |
|
PFN2 promotes proliferation and migration of ECs, as well as tube formation and reaches the maximum level in 10-day embryo. |
|
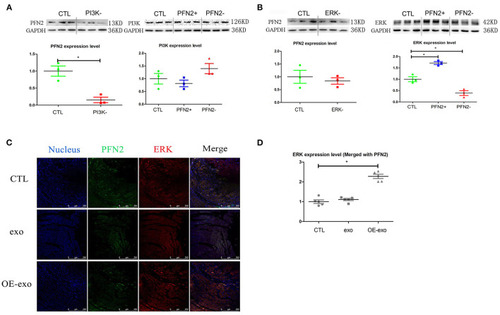
PFN2 involved molecular signaling pathway. |
|
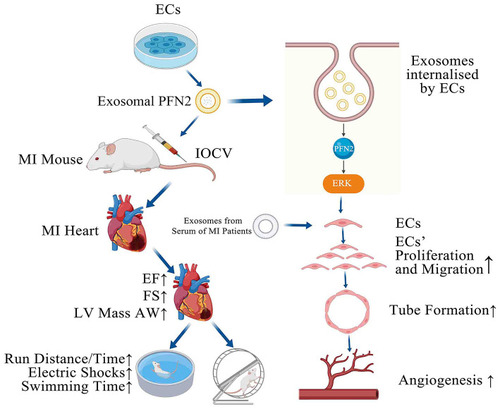
GRAPHICAL ABSTRACT. PFN2-overexpressing ECs (OE-exo) treated MI mice showed improvement in infarction volume, cardiac function and motor ability, and PFN2/ OE-exo significantly enhanced EC proliferation, migration, tube formation ability and angiogenesis. |