- Title
-
Steroid-sensitive nephrotic syndrome candidate gene CLVS1 regulates podocyte oxidative stress and endocytosis
- Authors
- Lane, B.M., Chryst-Stangl, M., Wu, G., Shalaby, M., El Desoky, S., Middleton, C.C., Huggins, K., Sood, A., Ochoa, A., Malone, A.F., Vancini, R., Miller, S.E., Hall, G., Kim, S.Y., Howell, D.N., Kari, J.A., Gbadegesin, R.
- Source
- Full text @ JCI Insight
|
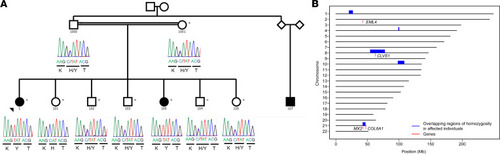
CLVS1 p.H310Y is a potential cause of familial childhood SSNS.
(A) Pedigree of family with SSNS showing segregation of the homozygous CLVS1 p.H310Y variant (c.928C>T, GRCH37/hg19) with disease. Filled circles and square represent affected individuals; unfilled circles, squares, and diamonds represent unaffected individuals. The parents of the family were estimated from their sequencing data to be second degree relatives (kinship coefficient of 0.106). (B) Regions of homozygosity (ROH) potentially implicated in SSNS. The 5 ROH (blue boxes, spanning each of the ROH on its respective chromosome) are those shared by both children with SSNS (ID 1 and 100) and are not in ROH in any of the unaffected relatives who were sequenced (ID 1000, 1001, 101). Gene locations are marked with red vertical lines. Candidate genes CLVS1 and MX2 are located in these ROH. |
|
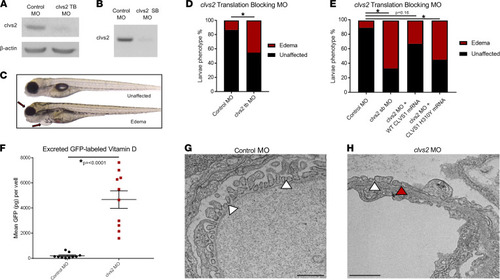
Knockdown of the clavesin gene in zebrafish (clvs2) results in edema phenotypes.
(A and B) Translation blocking and splice block morpholinos were used to knock down clvs2 expression in zebrafish. Morpholino efficacy was confirmed through Western blot (A) and reverse transcription PCR (RT-PCR) (B), showing that both morpholinos were able to knock down clvs2 expression. (C) Larval phenotypes were evaluated at 4 days postfertilization (dpf) as having edema (arrows for periorbital and pericardial) or as unaffected (no edema). (D and E) Analysis revealed significantly increased edema phenotypes with clvs2 knockdown compared with controls, and this edema could be rescued by coinjection of wild-type human CLVS1 mRNA but not the p.H310Y variant (E) (*P < 0.05, n > 60 for all groups, 1-way ANOVA). (F) Quantification of excreted GFP-labeled vitamin D in the Tg(lfabp:VDBP-GFP) reporter line revealed a loss of GFP in clvs2 morpholino-injected fish compared with controls (n = 10 for each group, P < 0.0001, 2-tailed t test), demonstrating that GFB integrity was affected by clvs2 knockdown. (G and H) Transmission electron microscopy images show healthy podocyte foot processes with intact slit diaphragms (white arrowheads) around a capillary loop in 5 dpf control morphant larvae and podocyte effacement (red arrowhead) in clvs2 morphants (scale bars = 800 nm and 1 μm, respectively), confirming that edema phenotypes are due to reduced GFB integrity. |
|
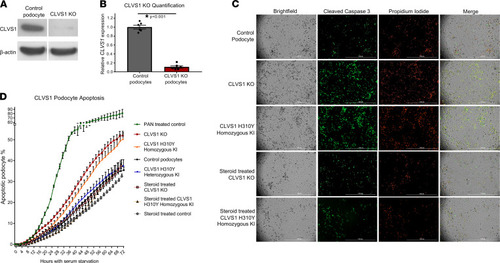
Loss of CLVS1 expression increases podocyte susceptibility to apoptosis that can be restored by corticosteroid treatment.
(A and B) Western blot showing significantly reduced expression of CLVS1 in CRISPR/Cas9-mediated knockout (KO) podocyte cell lines compared with control cell lines (n = 4, P < 0.001, 2-tailed t test). (C) Still images depicting cleaved caspase-3 activity (green) as reporter for early apoptosis and propidium iodide staining (red) for late apoptosis and necrosis in human podocytes 72 hours after serum starvation (scale bars = 1 mm). (D) Quantification of these images over time revealed an increase in podocyte susceptibility to serum starvation–induced apoptosis in CLVS1-KO and homozygous H310Y-knockin (KI) podocytes compared with controls (P < 0.05 for all time points after 16 hours for KO and after 34 hours for KI, n > 25 for each group, 2-way ANOVA). Heterozygous H310Y-KI podocytes displayed similar apoptotic phenotypes to controls. The elevated apoptosis in CLVS1-KO and homozygous H310Y-KI podocytes was rescued by treatment with 1 μM dexamethasone. |
|
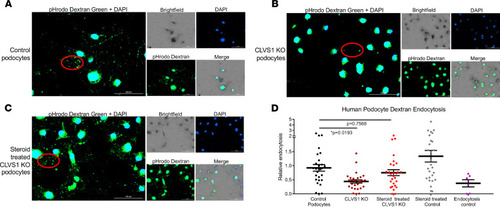
Loss of CLVS1 causes decreased podocyte endocytosis.
(A–C) Images showing the decreased endocytosis of fluorescently labeled dextran (pHrodo Dextran) in conditionally immortalized podocytes with CRISPR/Cas9-mediated KO of CLVS1 that can be restored with treatment with 1 μM dexamethasone (representative molecules circled in red). (D) Quantification of these images revealed a significant (P = 0.0193) loss of dextran endocytosis in KO podocytes compared with controls that was eliminated when these podocytes were treated with dexamethasone (P = 0.7568, n > 20 for each experimental group, 1-way ANOVA). A selective inhibitor of dynamin I and dynamin II, Dynasore, which reduces clathrin-mediated endocytosis, was used as an endocytosis control. Errors bars depict SEM. |
|
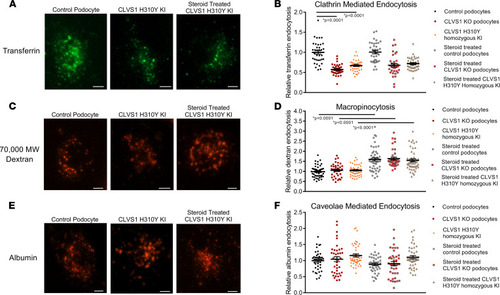
CLVS1 is required for clathrin-mediated endocytosis in human podocytes.
To examine specific modes of endocytosis in CLVS1 podocytes, we evaluated the internalization of fluorescently labeled transferrin (clathrin-mediated endocytosis), 70,000 MW dextran (macropinocytosis), and albumin molecules (caveolae-mediated endocytosis). (A and B) Quantification of the number of internalized transferrin molecules per cell revealed a decrease in clathrin-mediated endocytosis in CLVS1-KO and homozygous H310Y-KI podocytes compared with controls that was unaffected by pretreatment with 1 μM dexamethasone. (n > 30 for each group, P < 0.0001, 1-way ANOVA.) (C and D) Macropinocytosis, as measured by the internalization of fluorescently labeled 70,000 MW dextran molecules, was unaffected by CLVS1 KO or H310Y KI (P = 0.9572 and P = 0.9604 respectively, 1-way ANOVA). However, treatment with dexamethasone did significantly increase macropinocytosis in all cell lines compared with vehicle-treated controls (P < 0.001 for all). (E and F) Caveolae-mediated endocytosis was comparable among CLVS1-KO, homozygous H310Y-KI podocytes, and controls when internalized albumin molecules were quantified, and this was unaffected by treatment with dexamethasone (n > 30 for each group, P = 0.993 and P = 0.1844 respectively, 1-way ANOVA). Error bars depict SEM in graphs. Scale bars = 12 μm. Each dot in the graphs represents the mean number of molecules per podocyte for a single well. |
|
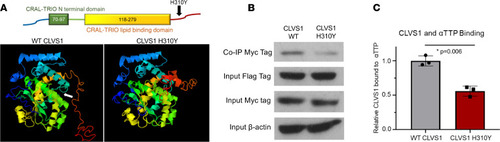
The CLVS1 p.H310Y variant decreases clavesin-1 ligand binding to α-tocopherol transport protein.
(A) The p.H310Y change is predicted to cause major structural alterations to the C-terminus of clavesin-1 and interfere with α-tocopherol–binding domain (white arrow). (B and C) Coimmunoprecipitation studies revealed a decrease in Myc-tagged clavesin-1 bound to the immunoprecipitated Flag-tagged αTTP when both are expressed at equivalent levels in HEK293 cells (n = 3, P = 0.006, 2-tailed t test). |
|
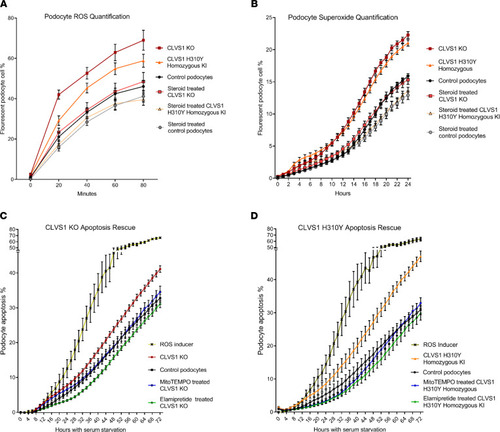
Defective ROS regulation contributes to decreased viability in CLVS1 KO and H310Y KI podocytes.
(A and B) Automated live-cell imaging and quantification of ROS levels in CLVS1-KO podocytes as well as controls using a fluorescent reporter of multiple ROS types, including hydrogen peroxide, peroxynitrite, and hydroxl radicals (A) (n > 20 for each group, P < 0.0001 for all time points for KO, P < 0.05 for all time points, and after 20 minutes for KI, 2-way ANOVA), as well as a second independent fluorescent reporter that detects superoxide generation (B) (n > 20 for each group, P < 0.001 for all time points after 10 hours, 2-way ANOVA), revealed an increase in ROS and superoxide accumulation in CLVS1-KO and homozygous KI podocytes that could be rescued with pretreatment with 1 μM dexamethasone. (C and D) The increased susceptibility to apoptosis in CLVS1-KO and homozygous KI podocytes (P < 0.05 for all time points after 36 hours) could be rescued by treatment with a superoxide scavenger or an ROS inhibitor (5 nM MitoTEMPO and 500 nM elamipretide) (n > 10 for all conditions, 2-way ANOVA). The ROS inducer pyocyanin (300 μM) and ROS inhibitor N-acetyl-l-cysteine (5 mM) were used as positive and negative controls, respectively, for all experiments. Error bars depict SEM. |
|
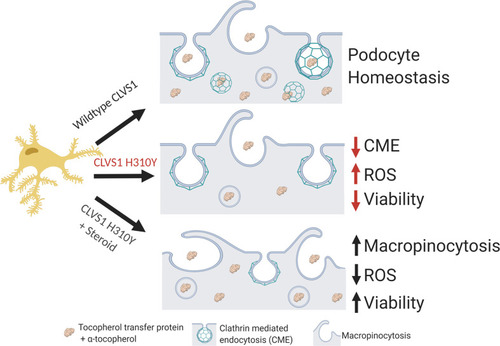
Summary figure.
Based on the data acquired in this study, we posit that CLVS1 function is required for CME in podocytes and disruptions in this process lead to disruption of GFB resulting in glomerular disease. The p.H310Y variant reduces the CME of key podocyte molecules, such as α-tocopherol, whose absence drives increased ROS and reduces viability. This pathological condition can be improved by the increased macropinocytosis and antioxidant enzyme activity in podocytes caused by corticosteroid treatment. |