- Title
-
Back and forth: History of and new insights on the vertebrate lymphatic valve
- Authors
- Shin, M., Lawson, N.D.
- Source
- Full text @ Dev. Growth Diff.
|
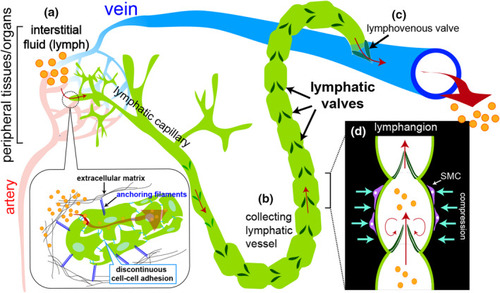
Schematic of fluid flow in the lymphatic system. (a) Interstitial fluid (referred to as lymph, orange circles) containing waste, small molecular lipoproteins and plasma leak from blood capillaries and are absorbed into lymphatic capillaries through discontinuous cell‐cell junctions. Here, lymphatic endothelial cells are held in place via anchoring filaments (blue lines) attached to surrounding extracellular matrix (black lines). (b) Lymph is transported from lymphatic capillaries into collecting lymphatic vessels where unidirectional lymph flow is maintained by numerous intraluminal lymphatic valves (dark green). (c) Collecting lymphatic vessels make direct connections with a vein through a lymphovenous valve to return fluid back to the circulatory system. (d) The region between successive lymphatic valves within the collecting vessel is referred to as a lymphangion. Collecting vessels exhibit smooth muscle cell coverage (SMC, purple cells) to aid in propulsion, while the bulk of flow is stimulated by the contraction of neighboring skeletal muscles |
|
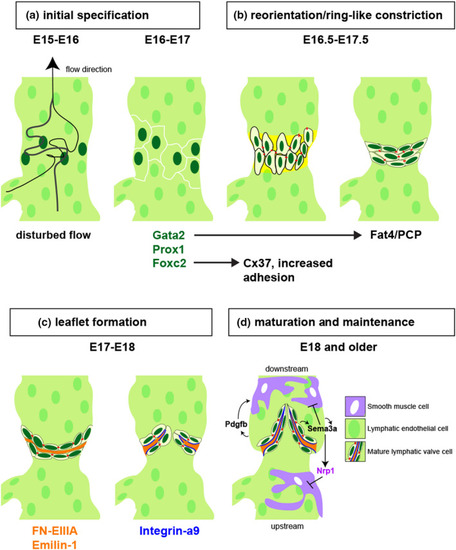
A schematic drawing of lymphatic valve development in collecting lymphatic vessel of mouse model adjusted and modified from (Koltowska et al., 2013; Tatin et al., 2013). (a) From E15~E16, disturbed lymph flow (from bottom to top) downstream from a branch point induces Foxc2 and Gata2 expression, which in turn induces Prox1 to initiate valve development at E16-E17 (Cha et al., 2018; Kazenwadel et al., 2015; Norrmén et al., 2009; Petrova et al., 2004; Qu et al., 2015; Sabine et al., 2012; Zhou et al., 2010). (b) From E16.5 to E17.5, key transcription factors stimulated by shear stress regulate cell-cell adhesion (e.g. Foxc2 and Cx37) and cell polarity (e.g. Gata2 and Fat4) to promote reorganization of lymphatic valve cells to form a ring-like constriction with expression of basement membrane proteins (yellow). (c) Between E17 and E18, LvEC secrete ligands for Integrin-a9 (orange, FN-EIIIA and Emilin-1) into an extracellular matrix core, and express Integrin-a9 on their basal surface (blue lines) to promote valve leaflet extension. (d) At E18.0 or later, lymphatic valves are fully mature and collecting lymphatic vessels exhibit smooth muscle coverage (SMCs, purple). SMCs are recruited by Pdgfb, except at the valve, where they are repelled by Sema3a binding to Nrp1, a direct target of Nrp1. Subcellular types in mature lymphatic valves are color-coded and listed |
|
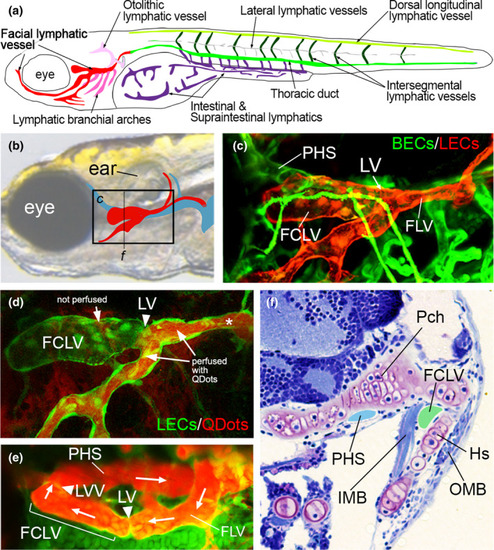
Larval zebrafish facial lymphatic vessel network. (a) Schematic drawing of main lymphatic networks of facial, lateral intestinal and trunk regions of the zebrafish larvae in lateral view. Lymphatic vessels in each network are classified and color-coded as indicated. (b) Schematic depicting location of facial lymphatic vessel (red) and primary head sinus (blue) on transmitted light image of a zebrafish larval head. Box denotes region in (c), dotted line indicates plane of section shown in (f). (c) Confocal image of blood vessels (blood vascular endothelial cells, BECs, express green fluorescent protein) and facial lymphatic vessel endothelial cells (LECs, red). PHS, primary head sinus; LV, lymphatic valve; FLV, facial lymphatic vessel. (d) Confocal lymphangiography of FCLV and FLV in zebrafish larva at 7 dpf. LECs express green fluorescent protein. Regions with and without QDot perfusion (red) are indicated. Asterisk denotes location of QDot injection. (e) Confocal lymphangiography depicting facial lymphatic vessels and PHS. Ventral view, anterior to the left. Position of the lymphovenous valve (LVV) and LV are shown. Arrows indicate direction of flow from FLV to FCLV and back into the PHS. (f) Transverse section of trypan blue-stained zebrafish larva. Lumens of PHS and FCLV are blue and light green, respectively. Frontal view, dorsal is up. HS, hyosymplectic cartilage; IMB, inner muscle bundle; OMB, outer muscle bundle; Pch, parachordal cartilage. Image courtesy of Sumio Isogai. Schematic illustration (a) is modified from Okuda et al. (2012), with permission. Schematic and images (b–d) are re-used from Shin et al. (2019), while (e) from Shin et al. (2016) with permissions |
|
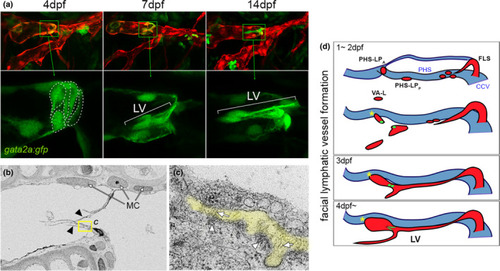
Cellular and ultrastructural features of zebrafish larval lymphatic valves. (a) Confocal images of facial lymphatic vessels at indicated time point. Lymphatic endothelial cells are in red, lymphatic valve (LV) cells also express the gata2a:gfp transgene in green. Bottom panels are magnified region indicated in corresponding top image; only green channel is shown. Valve cell shapes traced with dotted lines at 4 dpf. Lateral view, anterior to left, dorsal is up. (b) Scanning electron microgram of lymphatic valve leaflets (indicated by arrowheads). Mural cells (MCs) adjacent to lymphatic endothelial cells are shown. Yellow box indicates region magnified in (c). (c) Extracellular matrix (ECM) core between two lymphatic valve endothelial cells is pseudocolored in yellow. Fibril-bundles are shown by arrows and electron dense regions suggestive of matrix binding are denoted by arrowheads. (d) Schematic depicting facial lymphatic vessel (red) morphogenesis; primary head sinus (PHS) shown in blue. Lymphatic valve progenitors and valve shown in green. CCV, common cardinal vein; FLS, facial lymphatic sprout; FLV, facial lymphatic vessel; PHS-LPA, primary head sinus-anterior lymphatic progenitor; PHS-LPP, primary head sinus-posterior lymphatic progenitor. Images (a–c) are re-used from Shin et al. (2019) with permission |
|
Examples of lymphatic anatomy in cartilaginous and bony fish. Traced drawings depicting the anterior lymphovenous networks in (a) jawless fish (lamprey, after metamorphosis), (b) cartilaginous fish (shark) and (c) bony fish (trout). Dorsal views, lymphatic vessels depicted in yellow, veins in black, and arteries in red. (a, b) Cranial lymph propulsor (LP) and jugular veins are noted. Arrows indicate direction of flow. (c) Primary head sinus (PHS) and facial lymphatic vessel (FLV) assigned based on similar anatomical positions in larval zebrafish. Traced sketches after Kampeier, 1969 with permission |