- Title
-
Development and biological characterization of a clinical gene transfer vector for the treatment of MAK-associated retinitis pigmentosa
- Authors
- Tucker, B.A., Burnight, E.R., Cranston, C.M., Ulferts, M.J., Luse, M.A., Westfall, T., Scott, C.A., Marsden, A., Gibson-Corley, K., Wiley, L.A., Han, I.C., Slusarski, D.C., Mullins, R.F., Stone, E.M.
- Source
- Full text @ Gene Ther.
|
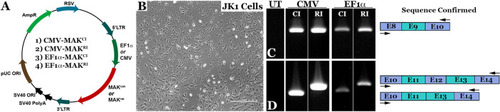
Design and generation of MAK gene transfer constructs.
A Schematic depicting the four AAV constructs generated in this study. Constructs were designed to deliver either the human canonical (MAKCI - lacks exon 12, constructs 1 and 3) or retina-specific MAK isoform (MAKRI - contains exon 12, constructs 2 and 4) under control of either the constitutively active CMV or EF1α promoter. B Representative image of cultured murine JK1 cells (immortalized SMA + , CD34 + testicular stromal cells, which lack endogenous expression of human MAK). Scale bar 100 μm. C, D rt-PCR analysis performed on RNA isolated from JK1 cells transduced with either AAV5-CMV-MAKCI (Construct 1), AAV5-CMV-MAKRI (Construct 2), AAV5-EF1α-MAKCI (Construct 3), or AAV5-EF1α-MAKRI (Construct 4). Each construct is capable of driving robust expression of exon-9-containing human MAK transcript (C). Unlike the MAKCI constructs, both AAV5-CMV-MAKRI and AAV5-EF1α-MAKRI constructs are capable of driving expression of the exon-12-containing retina-specific MAK transcript (D). Arrows denote rt-PCR primer positions. |
|
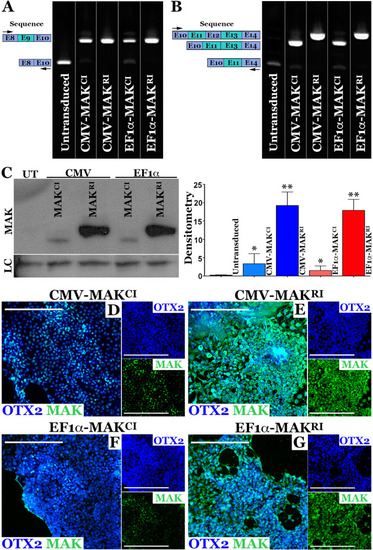
AAV5 mediated restoration of MAK transcript and protein in patient iPSC-derived photoreceptor precursor cells.
A, B rt-PCR analysis performed on RNA isolated from patient-specific photoreceptor precursor cells two-weeks following transduction with AAV5-CMV-MAKCI, AAV5-CMV-MAKRI, AAV5-EF1α-MAKCI, or AAV5-EF1α-MAKRI (MOI = 104vg/cell). Compared to untransduced cultures (UT), which lack expression of wildtype exon-9-containing MAK transcript, cells transduced with each of the AAV5 constructs expressed exon-9-containing MAK transcripts. Only AAV5-CMV-MAKRI and AAV5-EF1α-MAKRI were capable of driving expression of exon-12-containing retinal MAK (B). C Western blot of patient-specific photoreceptor precursor cells transduced with AAV5-CMV-MAKCI, AAV5-CMV-MAKRI, AAV5-EF1α-MAKCI, or AAV5-EF1α-MAKRI (MOI = 104vg/cell). Only the constructs driving MAK exon 12 restored expression of full-length retinal-specific MAK protein. D-G Immunocytochemical analysis of patient iPSC-derived photoreceptor precursor cells following AAV5 transduction using antibodies targeted against MAK and OTX2 (photoreceptor precursor cell marker). Although MAK was detected in cultures transduced with AAV5 vectors carrying the canonical isoform (AAV5-CMV-MAKCI (D) and AAV5-EF1α-MAKCI (F)), pronounced expression throughout the cell body and neurites was only detected in cultures transduced with vectors carrying the retinal isoform (AAV5-CMV-MAKRI (E) or AAV5-EF1α-MAKRI (G)). MAK – green, OTX2 – blue. Scale bars = 200 μm. Arrows denote rt-PCR primer positions. |
|
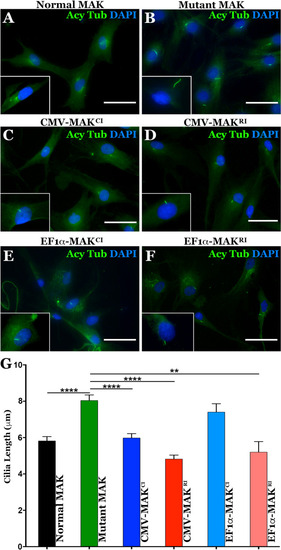
Restoration of MAK protein rescues aberrant primary cilia length defect in patient-derived dermal fibroblasts.
A–F Immunocytochemical analysis using an antibody targeted against anti-acetylated tubulin (primary cilia marker) to stain dermal fibroblasts isolated from an unaffected control individual (A – control) and a patient with molecularly confirmed MAK-associated RP (B–F). Cells from the patient with MAK-associated RP were transduced with viral vectors driving canonical (C, E) or retinal MAK (D and G. Untransduced cultures of patient derived cells were used as a disease phenotype control (B). G Histogram comparing mean primary cilia length between a normal individual (n = 25 cells) and 3 independent patients with MAK-associated RP before and after transduction with viral vectors driving either canonical or retinal MAK (n = 75 cells, 25 cells per patient for each of 3 patients). The primary cilium in fibroblasts isolated from patients with MAK-associated RP was significantly longer than that of an unaffected control individual. Transduction with retinal MAK under control of either the CMV or EF1α promoter reduced the primary cilium length to that of the control. A significant reduction in primary cilium length was detected following transduction of with canonical MAK driven by the CMV promoter only. (**p < 0.01, ****P < 0.001). Scale bars = 100 μm. |
|
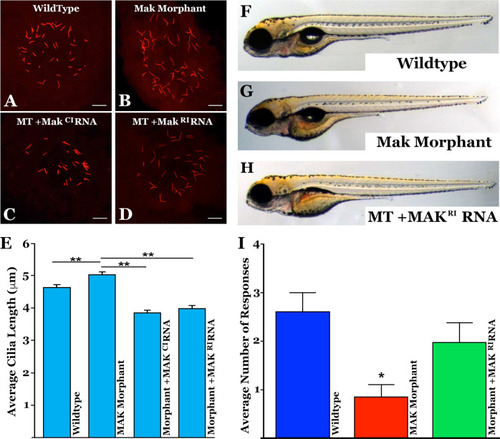
Injection of MAK mRNA restores Kupffer’s vesicle cilia length and response to visual stimulus in a mak mutant zebrafish model.
A–D Immunocytochemical labeling of primary cilia with an anti-acetylated tubulin antibody within Kupffer’s vesicle in uninjected (A), mak morpholino-injected (B), and morpholino-injected zebrafish that simultaneously received canonical MAKCI (D) or retinal MAKRI mRNA (D). E Quantification of mean cilia length measured in each treatment group shown in A–D. MAK morphants displayed significantly longer cilia compared to uninjected siblings (uninjected vs MO only; p = 0.0004). Sequential injection of retinal MAKRI mRNA or canonical MAKCI mRNA significantly shortened primary cilia length (MO only vs MO + RNA; p < 0.0001 for both). F–H Light micrographs of wildtype (F), MAK morpholino-injected (G), and MAK morpholino and retinal MAK mRNA injected (H) demonstrating normal overall morphology among groups. I Histogram depicting the number of responses in the vision startle assay of wild-type, MAK morpholino-injected (MAK Mutant) and MAK morpholino/MAKRI mRNA injected (Mutant + MAKRI mRNA). MAK morphants had a significant decrease in average number of responses compared to wild-type fish (Wildtype vs MAK Mutant; p < 0.05). Injection of retinal MAK mRNA into MAK mutants partially rescued visual responses (i.e. no significant difference between wild-type and treatment groups). Kruskal-Wallis test with Dunn’s multiple comparisons. Scale bars in A-D = 10 μm. PHENOTYPE:
|
|
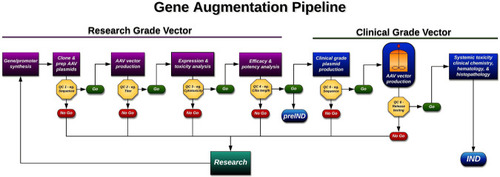
Schematic of therapeutic development pipeline described and followed in this study.
Using this strategy we were able to develop, test, and validate enough product to treat 500 patients (at a maximum clinical dose of 1 × 1011v.g.) with MAK associated RP for less than $500,000 USD (i.e., ~$1000 USD/patient). v.g. = viral genomes. QC = quality control. Example QC analysis performed at each step is provided. For a complete list of QC analysis performed see Supplemental Table 3. |