- Title
-
Defective Neuronal Positioning Correlates With Aberrant Motor Circuit Function in Zebrafish
- Authors
- Asante, E., Hummel, D., Gurung, S., Kassim, Y.M., Al-Shakarji, N., Palaniappan, K., Sittaramane, V., Chandrasekhar, A.
- Source
- Full text @ Front. Neural Circuits
|
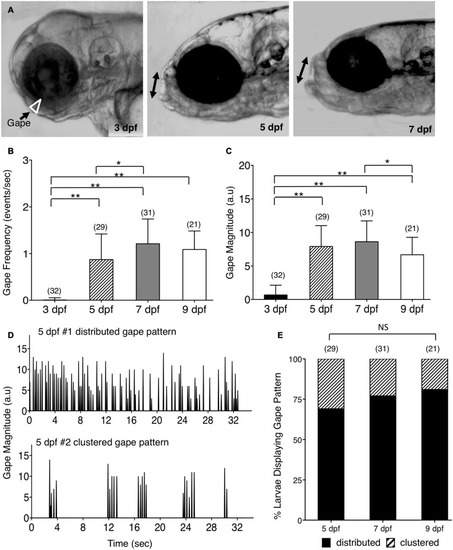
Ontogeny of lower jaw movement in wildtype zebrafish larvae. |
|
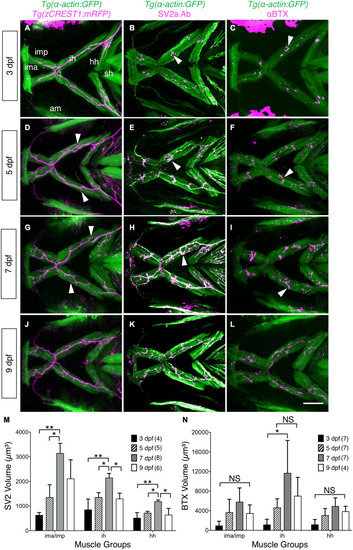
Developmental changes in branchiomotor axon branching and synaptic structures at the jaw neuromuscular junctions. Ventral views with anterior to the left of the jaw musculature. |
|
|
|
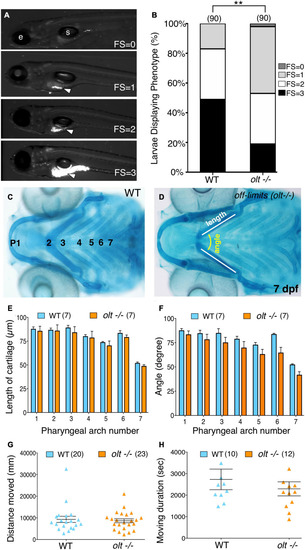
Defective jaw movements greatly reduce food intake in |
|
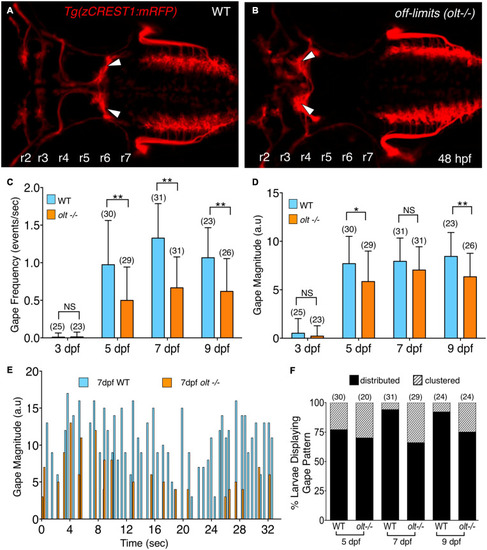
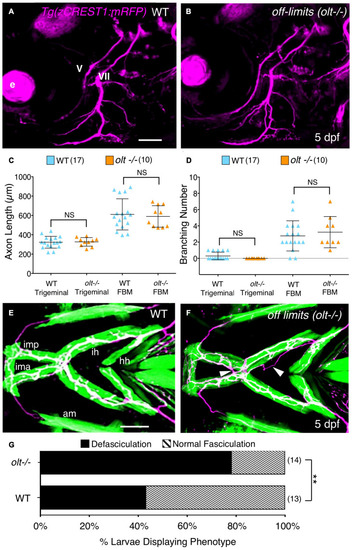
Axon guidance and outgrowth are unaffected in |
|
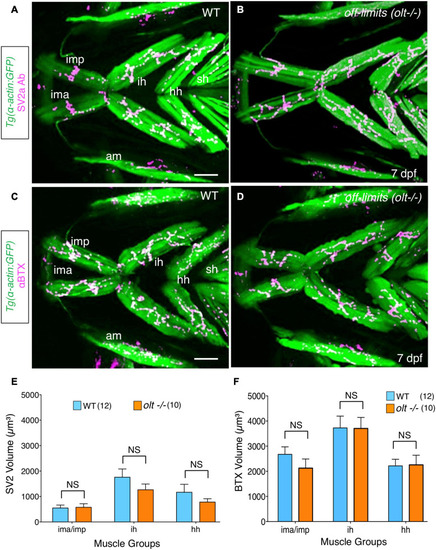
Neuromuscular junctions on jaw muscles in |
|
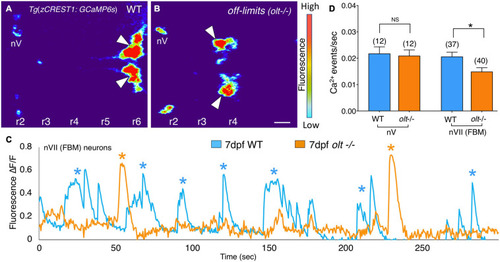
Facial branchiomotor neurons are less active in |