- Title
-
Drug screening with zebrafish visual behavior identifies carvedilol as a potential treatment for an autosomal dominant form of retinitis pigmentosa
- Authors
- Ganzen, L., Ko, M.J., Zhang, M., Xie, R., Chen, Y., Zhang, L., James, R., Mumm, J., van Rijn, R.M., Zhong, W., Pang, C.P., Zhang, M., Tsujikawa, M., Leung, Y.F.
- Source
- Full text @ Sci. Rep.
|
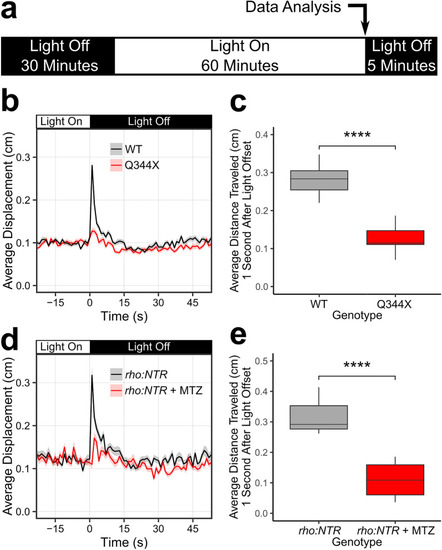
The Q344X larvae displayed a diminished scotopic light-off VMR driven by rods. ( |
|
Drug screening on the Q344X zebrafish identified carvedilol as a beneficial drug. ( |
|
Carvedilol treatment increased rod numbers in the Q344X larvae. Representative retinal cryosection of (a) a wildtype larva (WT), (b) a DMSO-treated Q344X larva, and (c) a carvedilol-treated Q344X (car) larva at 7 dpf. Rods were labeled by EGFP expression driven by rho promoter, and the nuclei were counterstained with DAPI. Scale = 50 μm. (d) Quantification of rod number in WT, DMSO-treated Q344X, and carvedilol-treated Q344X retinal cryosections from 5 to 7 dpf. There was a statistically significant difference in rod number between groups at all stages determined by one-way ANOVA at 5 dpf (WT, N = 11; Q344X, N = 16; F(1,25) = 71.04, p value < 0.0001), at 6 dpf (WT, N = 9; Q344X, N = 20; Q344X + car, N = 21 ; F(2,44) = 96.9, p value < 0.0001), and at 7 dpf (WT, N = 9; Q344X, N = 17; Q344X + car, N = 11; F(2,41) = 167.9, p value < 0.0001). The effect of Q344X rod degeneration and carvedilol treatment on rod number was assessed post hoc by pairwise t-test with false discovery rate correction at 6 dpf (WT − Q344X, p value < 0.0001; Q344X − Q344X + car, p value < 0.001) and at 7 dpf (WT − Q344X, p value < 0.0001; Q344X − Q344X + car, p value < 0.001). (e) Representative whole-eye images of WT, Q344X, and carvedilol-treated Q344X larvae at 7 dpf. Rods were labeled by EGFP expression. Left column: WT rods were mainly found on dorsal and ventral retina (top). They were abundantly present in the ventral patch of the retina extending medially (bottom). Middle column: Q344X rods were mostly degenerated at the same stage (top). There were only a handful of rods remaining near the lateral edge of the ventral patch in the Q344X retina (bottom). Right column: carvedilol treatment increased the number of Q344X rods on both dorsal and ventral retina (top); however, gaps of missing rods were still apparently on dorsal retina. More rods were observed in the ventral patch of the carvedilol-treated retina (bottom). Statistical analysis of whole-mount data is shown in Table 2. Scale = 100 μm. D dorsal, V ventral, M medial, L lateral. PHENOTYPE:
|
|
Carvedilol treatment beginning at 3 dpf increased rod numbers in the Q344X larvae greater than the treatment beginning at 5 dpf. (a) Quantification of rod number in WT, Q344X treated with DMSO beginning at 3 dpf, and Q344X treated with carvedilol beginning at 3 dpf or 5 dpf. Rods were quantified from their retinal cryosections beginning at 3–7 dpf. There was no statistically significant difference in rod number between groups at 3 dpf and 4 dpf determined by one-way ANOVA (3 dpf; N = 10; F(3,36) = 0.1, p value = 0.95); (4 dpf; N = 10; F(3,36) = 0.5, p value = 0.69). There was a statistically significant difference in rod number between groups at 5 dpf through 7 dpf determined by one-way ANOVA (5 dpf, N = 10; F(3,36) = 0.1, p value < 0.0001), (6 dpf, N = 10; F(3,36) = 0.1, p value < 0.0001), (7 dpf, N = 10; F(3,36) = 0.1, p value < 0.0001). The effect of Q344X rod degeneration and carvedilol treatment on rod number was assessed post hoc by pairwise t-test with false discovery rate correction at 5 dpf (WT − Q344X, p value < 0.0001; Q344X − Q344X + car3dpf, p value < 0.001; Q344X − Q344X + car5dpf, p value = 0.36, Q344X + car3dpf − Q344X + car5dpf, p value < 0.0001), at 6 dpf (WT − Q344X, p value < 0.0001; Q344X − Q344X + car3dpf, p value < 0.0001; Q344X − Q344X + car5dpf, p value < 0.05; Q344X + car3dpf − Q344X + car5dpf, p value < 0.05), and at 7 dpf (WT − Q344X, p value < 0.0001; Q344X − Q344X + car3dpf, p value < 0.0001; Q344X − Q344X + car5dpf, p value < 0.05; Q344X + car3dpf − Q344X + car5dpf, p value < 0.05). (b) Carvedilol treatment of Q344X larvae beginning at 3 dpf (purple trace) displayed a significant scotopic light-off VMR when compared to Q344X larvae treated with DMSO (red trace) (Hotellings T-squared test, N = 3 replicates of 24 larvae, T = 397, df = 30, p value < 0.0001). Each trace shows the average displacement of each replicate, and the color ribbons indicate µ ± s.e.m. |
|
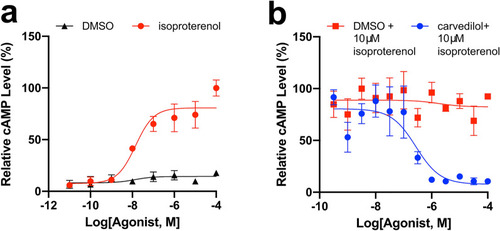
Carvedilol treatment might directly act on rods cells. To determine the extent to which carvedilol act directly on rods, we conducted a GloSensor cAMP assay with human Y79 cells. ( |