- Title
-
Loss of C2orf69 defines a fatal autoinflammatory syndrome in humans and zebrafish that evokes a glycogen storage-associated mitochondriopathy
- Authors
- Wong, H.H., Seet, S.H., Maier, M., Gurel, A., Traspas, R.M., Lee, C., Zhang, S., Talim, B., Loh, A.Y.T., Chia, C.Y., Teoh, T.S., Sng, D., Rensvold, J., Unal, S., Shishkova, E., Cepni, E., Nathan, F.M., Sirota, F.L., Liang, C., Yarali, N., Simsek-Kiper, P.O., Mitani, T., Ceylaner, S., Arman-Bilir, O., Mbarek, H., Gumruk, F., Efthymiou, S., Uğurlu Çi Men, D., Georgiadou, D., Sotiropoulou, K., Houlden, H., Paul, F., Pehlivan, D., Lainé, C., Chai, G., Ali, N.A., Choo, S.C., Keng, S.S., Boisson, B., Yılmaz, E., Xue, S., Coon, J.J., Ly, T.T.N., Gilani, N., Hasbini, D., Kayserili, H., Zaki, M., Isfort, R.J., Ordonez, N., Tripolszki, K., Bauer, P., Rezaei, N., Seyedpour, S., Khotaei, G.T., Bascom, C.C., Maroofian, R., Chaabouni, M., Alsubhi, A., Eyaid, W., Işıkay, S., Gleeson, J.G., Lupski, J.R., Casanova, J.L., Pagliarini, D.J., Akarsu, N.A., Maurer-Stroh, S., Cetinkaya, A., Bertoli-Avella, A., Mathuru, A.S., Ho, L., Bard, F.A., Reversade, B.
- Source
- Full text @ Am. J. Hum. Genet.
|
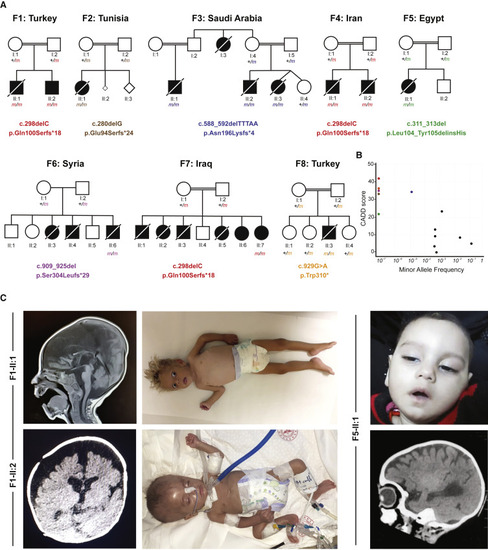
(A) Pedigrees of eight consanguineous families segregating homozygous C2orf69 loss-of-function variants. The identified germline homozygous mutations are shown for 12 affected individuals; eight additional children with similar symptoms died before they could be tested. (B) C2orf69 is intolerant of genetic variation. Minor allele frequency (MAF) and combined annotation-dependent depletion (CADD) score of homozygous C2orf69 coding variants found in gnomAD v.2.1.1 (black dots) and those found in each family (color-coded dots). (C) Photographs and brain MRIs taken at 10 months of age (F1-II:1), 5 months of age (F1-II:2), and 6 months of age (F5-II:1) showing cerebral atrophy with leukoencephalopathy. |
|
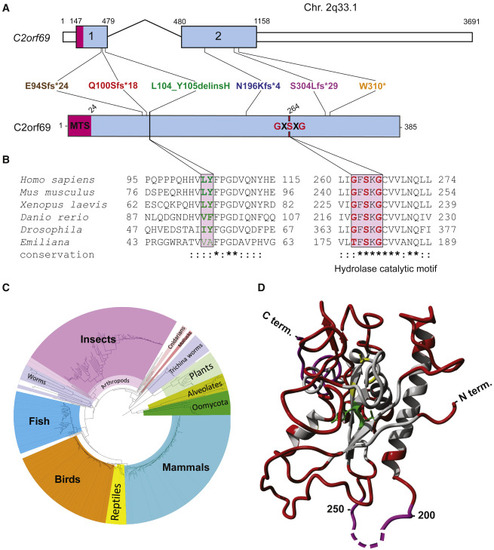
(A) Exon-intron genomic organization of C2orf69 with positions of the six germline loss-of-function mutations identified. (B) Protein organization of C2orf69 with positions of identified mutations. (C) Amino acid sequence conservation of C2orf69 orthologs across all major eukaryotic phyla. With the exception of fungi, C2orf69 is recorded in all metazoans, plants, and phytoplankton. (D) 3D structure prediction of human C2ORF69 with annotated residues L104_Y105 in green and predicted catalytic residue Ser264 in yellow. |
|
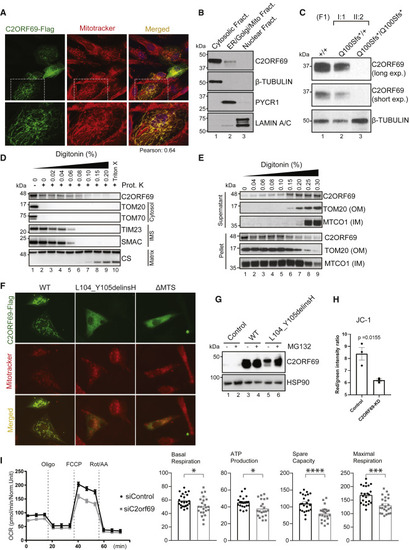
(A) Immunostaining of BJ-TERT fibroblasts overexpressed with C2ORF69-FLAG with FLAG antibodies (green) revealed co-localization with mitochondria marker, MitoTracker CXMRos (red). Pearson’s correlation coefficient was analyzed with Fiji software. (B) Cellular fractionation of primary fibroblasts indicates that endogenous C2ORF69 is mostly cytoplasmic, but a small fraction is associated with membranous fractions including mitochondria. (C) C2ORF69 protein is absent in primary dermal fibroblast with the homozygous p.Gln100Serfs∗18 variant. (D) Proteinase K protection assay of HEK293T mitochondria to reveal topology and submitochondrial location of endogenous C2ORF69. The majority of the protein resides in the outer membrane vulnerable to proteinase K, and a small fraction displays evidence of translocation into the mitochondria. IMS, intermembrane space. (E) Differential membrane extraction assays in HEK293T mitochondria confirm that endogenous C2ORF69 is both cytosolic and mitochondrial membrane associated. (F) Immunostaining of BJ-TERT fibroblasts overexpressed FLAG epitope-tagged wild-type (WT) C2ORF69, L104_Y105delinsH, and C2ORF69 without mitochondria-targeting signal (ΔMTS). We immunostained cells with FLAG antibodies (green) and MitoTracker CXMRos (red) to determine co-localization. (G) Immunoblot of fibroblasts overexpressing wild-type C2ORF69 or L104_Y105delinsH treated with proteasome inhibitor, MG132 (20 μM). Treatment with MG132 rescued expression level of p.Leu104_Tyr105delinsHis to near WT levels. (H) Ratiometric JC-1 (1 μM) membrane potential measurement in ReN VM neurons. Each dot represents the average of all ratiometric measurements across three separate 40× fields of one well. Data are mean ± SEM, and p value is derived from unpaired t test. (I) Agilent Seahorse Mito Stress Test on siControl- and siC2orf69-differentiated ReNcell VM neurons with basal respiration, ATP production, maximal respiration rate, and spare values indicated. |
|
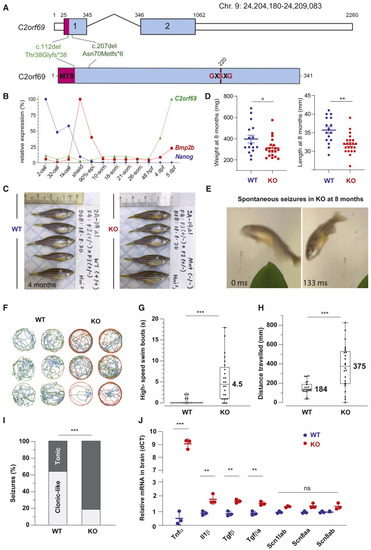
(A) Exon-intron structure of the C2orf69 ortholog in zebrafish. Annotations of the two distinct germline frameshift mutations generated by CRISPR-Cas9 editing at the genome and protein levels. (B) Developmental expression of C2orf69 during early zebrafish embryogenesis. The transcription of C2orf69 begins at 48 hpf without any detectable maternal contribution. (C) C2orf69 KO fish are indistinguishable from WT siblings at 4 months. (D) C2orf69 KO fish show statistically significant reduced body mass and length at 8 months. (E) Spontaneous seizures in adult 8-month-old KO fish lead to fully penetrant lethality. (F) Six representative swimming tracks extracted from 2 min videos of 11 dpf KO and WT larvae each show high-speed swimming bouts (red) within 5 min of exposure to 5 mM PTZ. (G) Quantification of high-speed swim bouts in 2 min (n = 24 each). The p value of the two-sided permutation t test is <0.0001 (H) Quantification of distance swam in 2 min (n = 24 each). The p value of the two-sided permutation t test is 0.0008 (I) The percentage of KO adult fish that show tonic seizures upon exposure to 5 mM PTZ (80%) within 12 min is 2.5-fold higher compared to WT (27%). The p value of the two-sided t test is 0.03. (J) Molecular markers from 4-month-old whole brain extracts measured by qPCR reveal constitutive CNS inflammation in KO adult fish compared to WT siblings. |
|
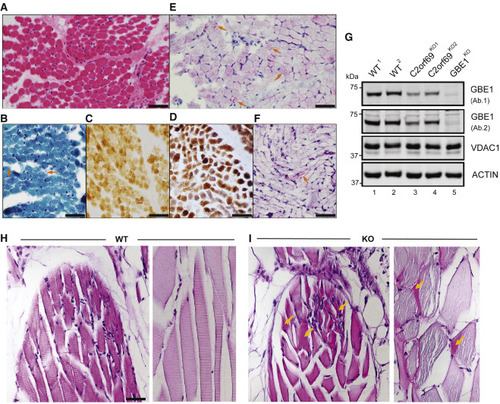
(A) Skeletal muscle biopsy of proband II:3 from family 8 shows mild variation of fiber size in H&E sections. (B) Subsarcolemmal accumulation of mitochondria is apparent in some fibers (arrows) by modified Gomori-trichrome stain. (C) Several fibers show faint staining by cytochrome-c-oxidase (COX) stain. (D) These COX-deficient fibers are more easily identified as bluish fibers by COX-SDH stain. (E) Periodic acid Schiff (PAS) stain shows unusual granular staining in many fibers (arrows), which are partially resistant to diastase, as seen by PAS-diastase stain (F). (F) PAS-positive material is seen in a few fibers after diastase treatment. Scale bars represent 50 μm. (G) Approximately 50% decrease in endogenous GBE1 levels is seen in two independent C2orf69 KO HAP1 cell lines with two different antibodies (Ab1, Proteintech; Ab2, Abcam). Two WT and GBE1 KO HAP1 cell lines act as positive and negative controls. Antibodies against VDAC1 and ACTIN are used as loading controls. (H) PAS stain in skeletal muscles of WT zebrafish at 6 months of age. (I) PAS-positive aggregates (marked by orange arrows) in skeletal muscles of C2orf69 KO zebrafish are suggestive of glycogen accumulation. Sarcomeres were partially disorganized in mutant striated muscle fibers. Scale bar represents 50 μm. |