- Title
-
Giantin is required for intracellular N-terminal processing of type I procollagen
- Authors
- Stevenson, N.L., Bergen, D.J.M., Lu, Y., Prada-Sanchez, M.E., Kadler, K.E., Hammond, C.L., Stephens, D.J.
- Source
- Full text @ J. Cell Biol.
|
|
|
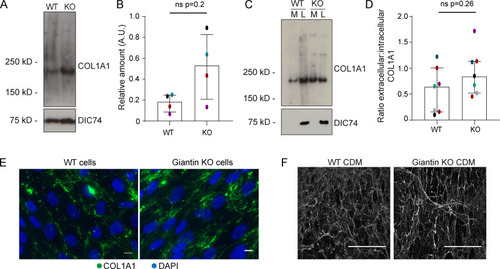
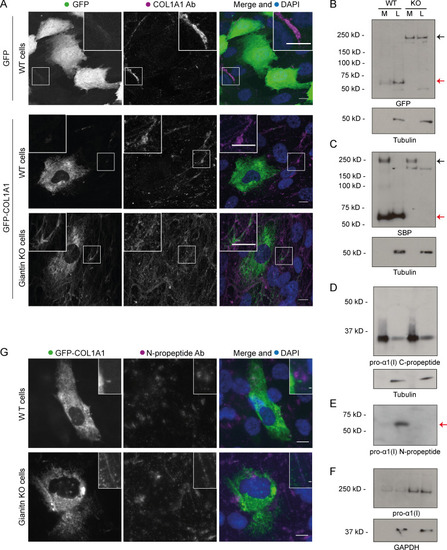
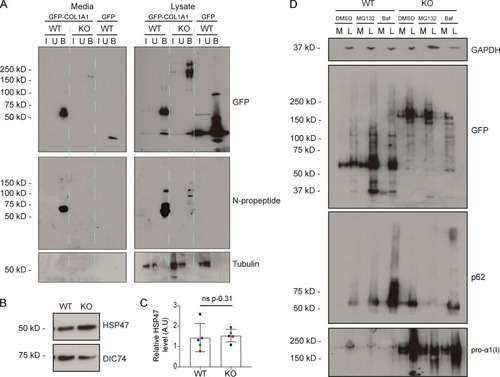
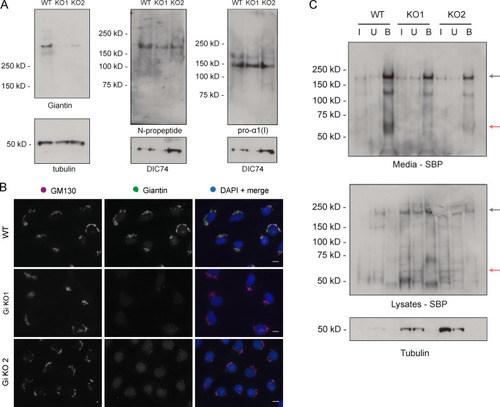
(A) Immunoblot of pro-α1(I) and dynein intermediate chain (DIC74, housekeeping) in cell lysates taken from WT and giantin KO cells. (B) Densitometry of the semiquantitative ECL immunoblots represented in A. Dots show individual replicates, and each independent experiment is color coded between cell lines. Bars depict median intensity of pro-α1(I) (COL1A1) normalized against DIC74 (n = 4 biological replicates). Error bars = interquartile range. Statistical test: Mann-Whitney U test. (C) Immunoblot of media (M) and cell lysate (L) fractions taken from WT and giantin KO cell cultures after 16-h incubation with serum-free medium plus 50 µg/µl ascorbate. (D) Ratio of extracellular versus intracellular levels of collagen as measured from secretion assays represented in C. pro-α1(I) (COL1A1) levels measured by densitometry from semiquantitative enhanced chemiluminescent blots and normalized against DIC74 before calculating ratios. Dots show individual replicates, and each independent experiment is color coded between cell lines. Bars depict median and interquartile range (n = 7 biological replicates). Statistical test: Mann-Whitney U test. (E) Maximum projections of widefield image stacks showing PFA fixed, unpermeabilized cells immunolabeled for endogenous pro-α1(I) (COL1A1, green). Nuclei are stained with DAPI (blue). Scale bars = 10 µm. (F) The cell-derived matrix produced by WT and giantin KO cells imaged as tilescans of confocal z-stacks of antibody-labeled pro-α1(I) presented as maximum projections. Scale bars = 2 µm (E) and 100 µm (F). A.U., arbitrary units. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|