- Title
-
Combinatorial glucose, nicotinic acid, and N-acetylcysteine therapy has synergistic effect in preclinical C. elegans and zebrafish models of mitochondrial complex I disease
- Authors
- Guha, S., Mathew, N.D., Konkwo, C., Ostrovsky, J., Kwon, Y.J., Polyak, E., Seiler, C., Bennett, M., Xiao, R., Zhang, Z., Nakamaru-Ogiso, E., Falk, M.J.
- Source
- Full text @ Hum. Mol. Genet.
|
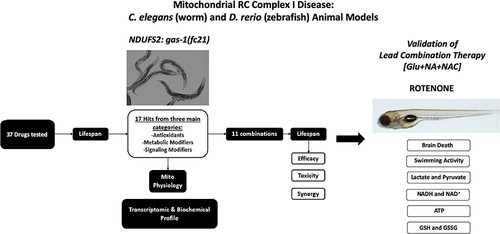
Schematic overview of experimental validation of combinatorial therapies in C. elegans and zebrafish models of respiratory chain complex I disease. Having previously identified 37 individual compounds in three major drug classes that significantly improved the short lifespan of complex I-deficient gas-1(fc21) mutant (that has a homozygous missense mutation in the nuclear-encoded complex I structural subunit NDUFS2 orthologue) C. elegans (worms) (Supplementary Material, Fig. S1), 11 combinations were randomly selected to evaluate the efficacy, toxicity and synergy of combining treatment classes on gas-1(fc21) worms’ lifespan as a primary outcome. Mechanistic analyses were performed at the level of mitochondrial physiology, transcriptome and metabolome analyses to interrogate the lead synergistic combination (glucose (Glu) + nicotinic acid (NAC) + N-acetylcysteine (NAC)). Validation of this lead combinatorial therapy was performed to assess brain death, swimming activity and effects on central biochemical readouts of mitochondrial dysfunction in a vertebrate zebrafish model of mitochondrial respiratory chain disease caused by pharmacologic (rotenone) inhibition of complex I function. |
|
Analysis of combinatorial treatments on C. elegans gas-1(fc21) complex I mutant worms’ lifespan. Lifespan analyses were performed at 20°C in gas-1(fc21) worms treated from the L1 early larval development stage with one of 11 randomly selected triple therapy combinations of three treatment classes that had previously been found to each significantly increase to variable degrees these complex I mutant worms’ lifespan (Supplementary Material, Figs. S2 and S3). All drug-treated lifespan analyses were performed relative to buffer-only treated gas-1(fc21) and N2 (WT) worms. (A-B) Lifespan results are shown for the 2 of 11 tested combinatorial therapy regimens that synergistically rescued gas-1(fc21) lifespan, even beyond that of WT worms. Key indicates number of worms studied across two biological replicate experiments per condition (n). Statistical analysis was performed by log-rank (Mantel-Cox) test comparing N2 (buffer only) or gas-1(fc21) worms treated with the indicated combination relative to buffer-only treated gas-1(fc21) worms. Subsequent analyses indicated that apparent efficacy of combination #6 (resveratrol + folinic acid + cysteamine bitartrate) was driven by resveratrol effect, without further synergy from additive therapies tested. (C) Glu + NA + NAC in triple or pairwise combinations showed synergistic rescue effect of gas-1(fc21) worms’ median lifespan relative to each individual component(s). Results convey median lifespan normalized to buffer-only exposed gas-1(fc21) worms. Detailed results are shown in Supplementary Material, Fig. S4. |
|
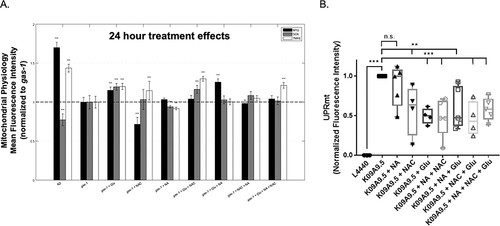
Glu + NA + NAC combinatorial treatment effects on C. elegans gas-1(fc21) worms’ mitochondrial physiology. (A) In vivo fluorescence analyses of mitochondrial content, mitochondrial oxidant burden and mitochondrial membrane potential were performed by terminal pharyngeal bulb (PB) relative fluorescence microscopy quantitation of MitoTracker Green FM (MTG), MitoSOX (SOX) and TMRE, respectively. gas-1(fc21) mutant synchronized young adult worms were treated for 24 h with Glu + NA + NAC combination therapy, or its individual and pairwise components, were compared with buffer-only treated gas-1(fc21) and N2 (WT) worms, and normalized to buffer-only gas-1(fc21) worms. Significant differences in mean fluorescence intensity between strains under different experimental conditions were assessed by mixed-effect ANOVA to account for potential batch effect due to samples being experimentally prepared, processed and analyzed on different days by including a batch random effect in the model. Statistical significance threshold was set at P < 0.05, and statistical analyses were performed in SAS 9.3. P-value conveys the significance of the difference between untreated N2 and untreated gas-1(fc21) (strain effect) or the difference between gas-1(fc21) plus drug(s) and untreated gas-1(fc21) (treatment effect). For each parameter, each drug treatment assay was repeated in 3 to 10 independent trials, with n = 50 worms per trial. Bars and error bars convey mean ± SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001 versus concurrent gas-1(fc21) buffer control. (B) Feeding RNA interference (RNAi) knockdown of gas-1 (K09A9.5) in an hsp-6p::gfp labeled wild-type reporter worm strain resulted in significantly elevated UPRmt in first day young adult stage worms relative to empty vector (L4440) RNAi control, which was significantly reduced by treatment from early development (L1 stage) with glucose (Glu) or N-acetylcysteine (NAC) and any pairwise or triple combinatorial regimens in which they were present. Nicotinic acid (NA) alone did not reduce UPRmt in this model. hsp-6p::gfp C. elegans was used as a positive control. All tests were carried out using three biological replicate independent trials, with approximately 300 worms per condition in each replicate. Boxes depict 10th and 90th percentile for the normalized fluorescence intensity. Whiskers depict minimum and maximum values for each condition. Statistical analysis was performed by unpaired t-test (**, P < 0.01; ***, P < 0.001) for each comparison, as indicated. |
|
Overall transcriptome dysregulation at the level of KEGG biochemical pathways was normalized by Glu + NA + NAC pairwise combinatorial therapies in C. elegans gas-1(fc21) worms. Parametric analyses of gene set enrichment (PAGE) scores are shown for selected KEGG pathways that showed significant dysregulation in gas-1(fc21) worms compared to N2 (WT) at baseline, and/or showed significant modulation in gas-1(fc21) worms when treated with Glu + NA + NAC triple combination therapy, or its individual or pairwise components, for 24 h at the young adult stage. For each comparison, the increased or decreased expression is shown for the second group named in the title relative to the first group. The greatest overall normalization of dysregulated pathway-level expression in gas-1(fc21) worms occurred with the 3 pairwise drug comparisons studied, * P < 0.05, **, P < 0.01, and ***, P < 0.001 for each two-way comparison, as shown. Additional transcriptome results are shown in Supplementary Material, Fig. S5. |
|
Combinatorial Glu + NA + NAC therapy partially restored altered intermediary metabolism of gas-1(fc21) mutant C. elegans. Whole worm concentrations and stable isotopic enrichment in free amino and organic acids of gas-1(fc21) worms were evaluated in synchronous young adult populations treated for 24 h with Glu + NA + NAC (glucose, nicotinic acid, and n-acetylcysteine) triple combination therapy or its single components and their pairwise combinations. (A) Whole worm free amino acid concentrations were measured by HPLC and normalized to overall protein concentration (nmol/mL per mg protein) for glutamic acid (GLU), glutamine (GLN) and alanine (ALA). Symbol color denotes experimental condition as defined in key, with separate symbols shown to indicate results of three independent replicate experiments performed. Random effects ANOVA statistical analysis was used to account for experimental batch effects. * P < 0.05 when compared to buffer (water) only exposed gas-1(fc21) worms. Results for additional HPLC amino acid levels are shown in Supplementary Material, Fig. S6. (B) Absolute isotopic enrichment of each amino acid metabolite in all molecular species for glutamate (GLU +2, +3, +4 and + 5), glutamine (GLN +2, +3, +4 and + 5), and alanine (ALA+2 and + 3) (indicating the number of carbon atoms on which 13C enrichment was present from U-13C6-glucose fed to the worms) was quantified by GC/MS in synchronous adult worm populations following U-13C6-glucose feeding to N2 (WT) or gas-1(fc21) worms for 24 h on the first day of adulthood. gas-1(fc21) worms were maintained in buffer (water)-only conditions or with Glu + NA + NAC as triple, pairwise, or single component therapies for 24 h, and compared to untreated N2 (WT) worms. Three biological replicate experiments were performed * P < 0.05 and ** P < 0.01 when compared to buffer (water) only exposed gas-1(fc21) worms. Results for additional GC/MS analytes are shown in Supplementary Material, Figs. S7–S8. |
|
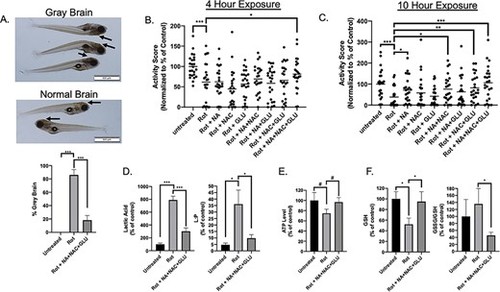
Combination Glu + NA + NAC treatment protected zebrafish larvae from rotenone-induced brain death, reduced swimming activity, and key biochemical hallmarks of mitochondrial complex I dysfunction. (A) Brain death (gray brain, indicated by arrows) was induced with 150 nM rotenone exposure in wild-type (AB) fish for approximately 5 h on 7 days post-fertilization (dpf). Pre-treating wild-type (AB) zebrafish beginning at 5 dpf with combinational GLU + NA + NAC therapy significantly prevented the brain death (golden color of brain, indicated by arrows) upon co-exposure to 150 nM rotenone at 7 dpf and combinational treatment for 5 h (P < 0.001). Three independent biological replicates were performed per condition, with n ≥ 15 animals in each condition per replicate. (B) Zebrafish larval swimming activity was quantified by exposure for 10 h to repetitive light cycles of 60% light for 20 min followed by 0% light for 20 min during rotenone co-exposure at 7 dpf. Independent experimental data was collected by ZebraBox (Viewpoint Life Sciences) analysis, with activity scores in the first 5 min of each dark period averaged across three independent biological replicate experiments with data normalized as percent of wild-type buffer-only control for each independent biological replicate (see Supplementary Material, Fig. S9). Effects of pre-treatment with Glu + NA + NAC triple combination therapy, along with each of the single and double permutations were evaluated, with activity score shown after rotenone (150 nM) co-exposure for 4 h, to assess biochemical mechanism before the 5-h peak when maximal brain death was observed. Data are represented in a scatter plot, with n = 8 animals per condition studied in each replicate. (C) Zebrafish larval swimming activity was quantified following pre-treatment with Glu + NA + NAC triple combination therapy, along with each of the single and double permutations, with activity score shown after 10 h co-exposure to rotenone (150 nM). n = 8 zebrafish larvae per condition in each replicate. (D) Zebrafish lactate and lactate:pyruvate ratios. Lactate levels were quantified in wild-type (AB) zebrafish (20 fish per replicate for each condition) in buffer or after rotenone exposure for 4 h either with or without Glu + NA + NAC pre-treatment from 5 dpf. Significant differences were seen between all treatment group (***, P < 0.001). Specifically, lactic acid mean levels were 279 pmol/larvae in buffer-only control larvae (n = 6 biological replicates), 2202 pmol/larvae in 150 nM rotenone ×4 h exposed larvae (n = 4 biological replicates), and 841 pmol/larvae in Glu + NA + NAC pre-treated rotenone ×4 h exposed larvae (n = 6 biological replicates). Pyruvate levels were not significantly changed between groups (Supplementary Material, Fig. S9). However, lactate-to-pyruvate (L:P) ratio was significantly increased in rotenone-exposed larvae and partially normalized with Glu + NA + NAC pre-treatment (*, P < 0.05). (E) ATP levels. Wild-type (AB) zebrafish (20 fish per replicate for each condition) pre-treated from 5 dpf with GLU + NA + NAC combinational therapy (n = 6 biological replicates) showed a trend toward improved ATP concentration upon evaluation after 4h rotenone exposure at 7 dpf as compared to rotenone (150 nM) exposed larvae for 4 h at 7 dpf without pre-treatment (n = 4 biological replicates). Specifically, ATP mean concentration in untreated AB zebrafish was 346 pmol per larvae, in rotenone-exposed AB zebrafish was 259 pmol per larvae, and in Glu + NA + NAC pre-treated zebrafish before rotenone exposure was 335 pmol per larvae. #, P ≤ 0.1. (F) Glutathione levels. Wild-type (AB) zebrafish (20 fish per replicate for each condition) exposed to 150 nM rotenone for 4 h (n = 7 biological replicates) had lower GSH mean concentration (85 pmol per larvae) than buffer-only control larvae (161 pmol per larvae (n = 6 biological replicates). Pre-treatment from 5 dpf with GLU + NA + NAC combinational therapy (n = 7 biological replicates) normalized GSH level (153 pmol per larvae), similar to that seen in buffer-control wild-type larvae. Glu + NA + NAC pre-treatment also significantly lowered the GSSG/GSH ratio relative to rotenone-only exposure without pre-treatment. Statistical significance between experimental conditions was assessed by Student’s t-test in Graphpad Prism 7.04, #, P ≤ 0.1, *, P < 0.05, **, P < 0.01, and ***, P < 0.001. Bars indicate mean and standard error of mean. |
|
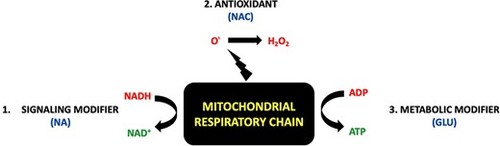
Schematic rationale supporting use of Glu+NA+NAC multi-drug combinatorial treatment regimen to replace major metabolite deficiencies in primary mitochondrial respiratory chain complex I disease. Understanding treatment approaches for the biochemical sequelae of respiratory chain (RC) disease may be simplified by reducing the RC to a ‘bioenergetic factory’ (black box). While the RC is the major site at which chemical energy is produced in the form of adenosine triphosphate (ATP), the RC also generates other several other essential ‘products’ needed to support normal cellular function. These include (A) nicotinamide adenine dinucleotide (NAD+) as generated in mitochondria by conversion of NADH-reducing equivalents generated through intermediary metabolism that are donated at CI (official enzyme name of CI is ‘NADH dehydrogenase’), and (B) oxidants, which are generated in mitochondria both with the mitochondrial matrix (via CI) and in the intermembrane space (via CIII). O., superoxide. H2O2, hydrogen peroxide. This simplified RC product view highlights major categories of therapies for RC dysfunction (red font), including (1) NAD+ supplementation (e.g. NA, nicotinic acid) (13), (2) antioxidants (e.g., NAC, to scavenge oxidants within mitochondria or throughout the cell) (14), and (3) optimizing production of ATP via anaerobic glycolysis (e.g. glucose or diet changes, to optimize cellular nutrition and therapeutically exploit upregulated glycolytic capacity). As shown by experimental modeling in C. elegans and zebrafish animal models of complex I disease, Glu + NA + NAC combinatorial therapies to correct all of these metabolite deficiencies that occur downstream of complex I dysfunction synergistically improves animal survival and health outcomes at diverse levels including mitochondrial physiology, intermediary metabolism, cellular signaling, stress resistance and overall animal activity. |