- Title
-
Improving CRISPR/Cas9 mutagenesis efficiency by delaying the early development of zebrafish embryos
- Authors
- Terzioglu, M., Saralahti, A., Piippo, H., Rämet, M., Andressoo, J.O.
- Source
- Full text @ Sci. Rep.
|
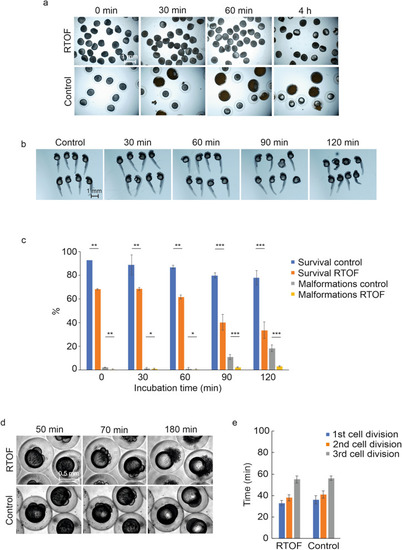
The effect of RTOF treatment on the oocyte viability and the early development of zebrafish embryos. ( |
|
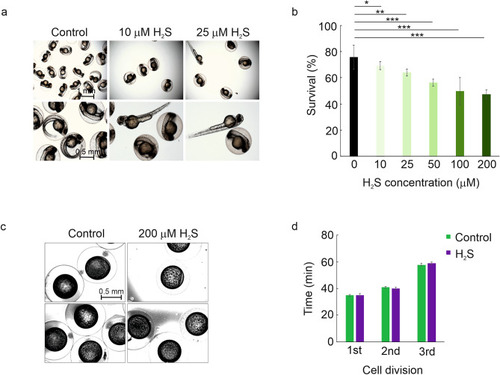
The effect of H2S treatment on the early development of zebrafish embryos. ( |
|
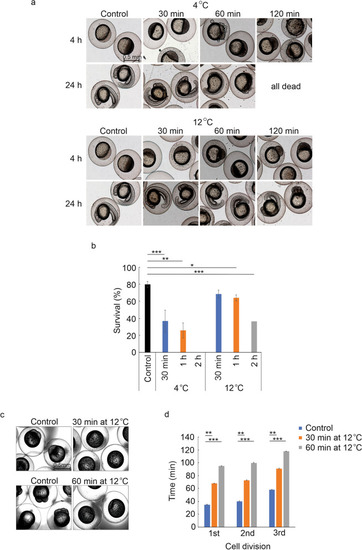
The effect of low temperatures on the early development of zebrafish embryos. ( |
|
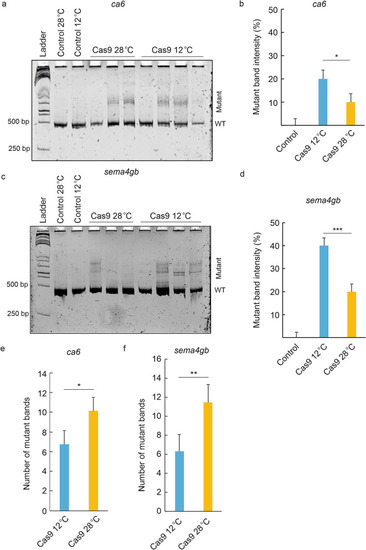
The effect of cold treatment on CRISPR/Cas9 mutagenesis efficiency in zebrafish embryos. Incubating zebrafish embryos in 12 °C immediately after Cas9/sgRNA injections increases the mutagenesis efficiency compared to embryos incubated in 28 °C. ( |