- Title
-
De novo enteric neurogenesis in post-embryonic zebrafish from Schwann cell precursors rather than resident cell types
- Authors
- El-Nachef, W.N., Bronner, M.E.
- Source
- Full text @ Development
|
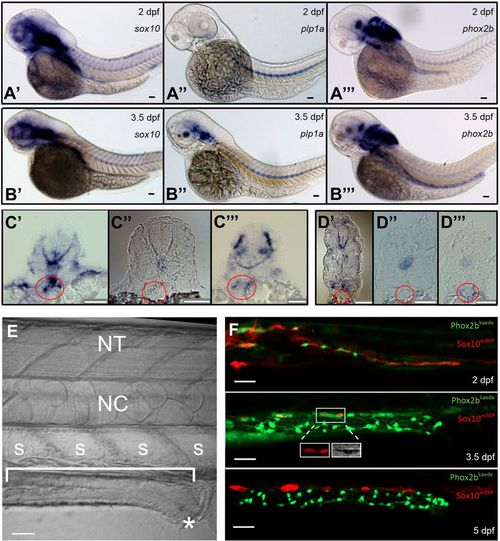
Resident neuronal progenitors are absent in the post-embryonic intestine. (A) ISH of enteric neural elements in 2 dpf embryos. sox10 is detected at 2 dpf as a stream in the midgut that does not yet extend to the hindgut (A′), plp1a exhibits no expression (A″) and phox2b has weak expression in the proximal gut (A‴). Expected probe trapping is evident in the notochord. (B) ISH of enteric neural elements in 3.5 dpf larvae. sox10 signal is absent in the intestine, although proximal probe trapping is seen in the nascent swim bladder (B′). plp1a is expressed dorsally but no expression is evident in the intestine (B″). phox2b expression extends throughout the intestine (B‴). (C′-C‴) Proximal cross-sections of 2 dpf embryos stained for sox10 (C′), plp1a (C″) and phox2b (C‴), with the nascent foregut marked by a red circle. (D′-D‴) Distal cross-sections of 3.5 dpf larvae stained for sox10 (D′), plp1a (D″) and phox2b (D‴), with the developing midgut marked by a red circle. (E) Anatomical orientation: fluorescent figures are oriented in this manner unless otherwise stated. The intestine is located ventrally (bracket) and extends anteriorly (left) to posteriorly (right), ending at the anus (*). A row of polygonal somites (S) are arranged dorsal to the intestine. The notochord (NC) and neural tube (NT, not visible in this image) are located dorsally. (F) Live imaging of Phox2b-kaede×Sox10-mRFP fish are consistent with ISH results: a migrating chain of Sox10 cells is observed in the midgut that does not yet extend to the hindgut at 2 dpf, but then Sox10 expression ceases at 3.5 dpf and 5 dpf. A few Sox10-expressing cells, seen dorsolateral to the intestine, are consistent with melanocytes, as supported by visible pigment using the transmitted light photomultiplier tube (TPMT), a detector for transmitted light (inset, 3.5 dpf panel). Scale bars: 50 µm. |
|
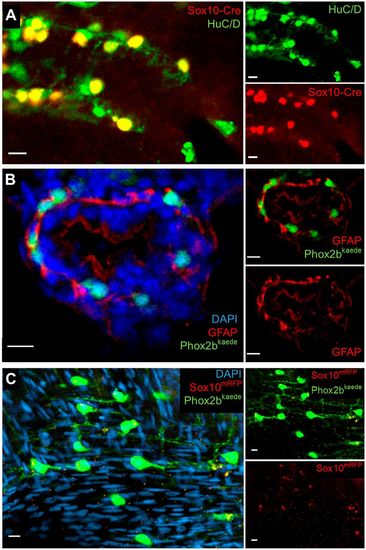
Further assays in larvae and adults support an absence of resident neuronal progenitors and enteric glia in the intestine. (A) Lineage tracing with an indelible Sox10-Cre line suggests enteric neurons are the sole fate of enteric vagal neural crest cells. At 5 dpf, fish were fixed and underwent IHC for the Cre reporter, mCherry and the neuronal marker HuC/D. All Cre-labelled cells colocalized with HuC/D, and no Cre+, HuC/D− cells were observed. (B) IHC with GFAP does not demonstrate convincing enteric glial cell bodies. Phox2b-kaede fish were fixed at 5 dpf, and axially sectioned for IHC for GFAP, a glial marker with cytosolic expression. Imaging of the endogenous Phox2b-kaede signal in concert with the GFAP IHC revealed a fibrillary pattern of GFAP closely associating with enteric neurons and other cells, which probably represents projections from extrinsic glia or nonspecific binding to other fibrillary proteins. (C) Whole-mount imaging of adult zebrafish intestine suggests that enteric glia and resident neuronal progenitors are not detectable by classical criteria later in development. Adult intestine from Phox2b-kaede×Sox10-mRFP fish that underwent optical clearing with RIMS revealed numerous enteric neurons, but no cell bodies expressing Sox10. Extrinsic glial projections are suggested by a fibrillary pattern of Sox10 expression. Scale bars: 10 µm. |
|
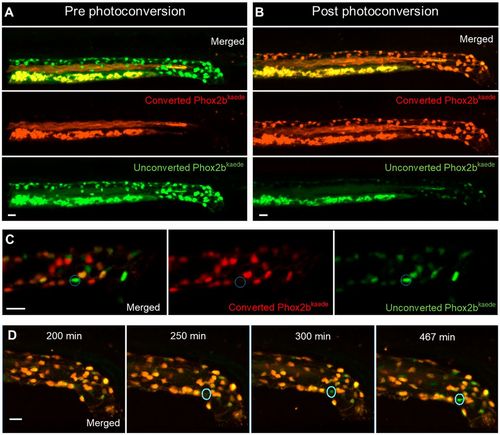
Enteric neurogenesis persists in the post-embryonic development despite the apparent absence of resident neuronal precursors. (A,B) 2D projection of z stack from a 4.5 dpf Phox2b-kaede fish demonstrates green fluorescent enteric neurons, but no red fluorescent cells (A). Yolk and intraluminal mucous exhibit expected autofluorescence in both channels. After photoconversion of all Phox2b-kaede neurons in the gut, all enteric neurons fluoresce red, although some retain decreased green fluorescence (B). (C) Live imaging 12 h after photoconversion at 4.5 dpf reveals the appearance of green fluorescent enteric neurons in the intestine with no red fluorescence (a representative neuron is circled), indicating that these neurons did not arise from pre-existing red fluorescent Phox2b-kaede cells. (D) Live 2D projection of a 10 h time-lapse after photoconversion at 4.5 dpf detects the emergence of de novo enteric neurons (circled), as indicated by the gradual appearance of a green-only neuron in a region of the intestine that was originally not occupied by a neuron. Scale bars: 20 µm. |
|
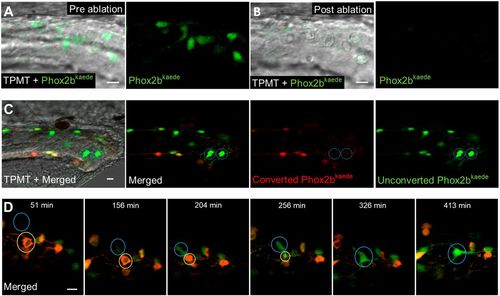
De novo enteric neurons replace ablated neurons in a post-embryonic injury model. (A,B) Prior to two-photon laser ablation, Phox2b-kaede enteric neurons are clearly visualized within the hindgut (A). After ablation, these neurons are no longer present in the hindgut, and TPMT reveals the injury site to be restricted to the neuron location (B). (C) At 4.5 dpf, fish underwent laser ablation of ten enteric neurons within the distal hindgut, followed by photoconversion of all remaining enteric neurons within the whole length of the gut. Live imaging was performed 12 h later and detected multiple de novo green fluorescent-only enteric neurons in the hindgut. (D) 8 h time-lapse of a fish at 4.5 dpf that underwent focal injury (but not complete ablation) of enteric neurons followed by pan-gut photoconversion reveals the involution of an injured neuron (yellow circle) that is replaced by a de novo green fluorescent-only enteric neuron. The new neuron (blue circle) initially appears very faintly at the dorsalmost aspect of the intestine but increases in intensity as it migrates to replace the involuted neuron and extends projections to neighbouring neurons. Scale bars: 10 µm. |
|
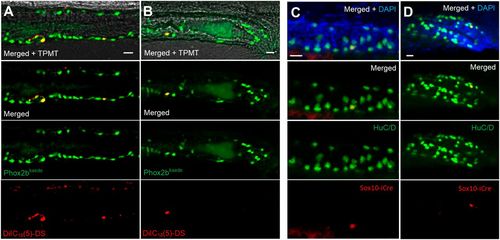
Lineage tracing demonstrates a trunk neural crest origin of post-embryonic neurons. (A,B) Phox2b-kaede embryos underwent neural tube injections of a far-red lipophilic dye at 30 hpf, after the vagal crest has delaminated from the neural tube. Live images at 6 dpf of the midgut (A) and hindgut (B) demonstrate Phox2b-kaede enteric neurons that colocalize with the dye, indicating their trunk origin. (C,D) Fish from the inducible Sox10-Cre line were exposed to 4-OHT at 3.5 dpf and underwent IHC for the Cre reporter, mCherry and the neuronal marker HuC/D at 5.5 dpf, with Cre-labelled enteric neurons observed in the midgut (C) and hindgut (D). As Cre induction occurring after Sox10 is no longer present within the intestine, these results support a trunk neural crest origin of these enteric neurons. Scale bars: 20 µm. |
|
5HT4R is present in the zebrafish intestine and prucalopride increases intestinal motility. (A,B) Transverse section of 5 dpf IHC for 5HT4R reveals diffuse expression within the intestinal epithelium as well as occasional neurons that colocalize with this label (A). Longitudinal sections demonstrate diffuse expression in the proximal foregut and additionally reveal 5HT4R+ projections spanning the dorsoventral axis (B). (C) Stills from a video of a live HuC-H2B GCaMP6 fish exposed to 10 µM prucalopride at 5 dpf reveal increased intestinal motility, as measured by expulsive contractions of autofluorescent intraluminal mucous into the external environment (0.5 versus 4.0; P=0.0004). Increased GCaMP signal was observed in association with expulsive contractions, suggesting neuronally mediated motility. (D) Fish exposed to prucalopride exhibited significantly more expulsive contractions compared with controls. Data are mean±s.e.m. Scale bars: 20 µm (inset, 5 µm). |
|
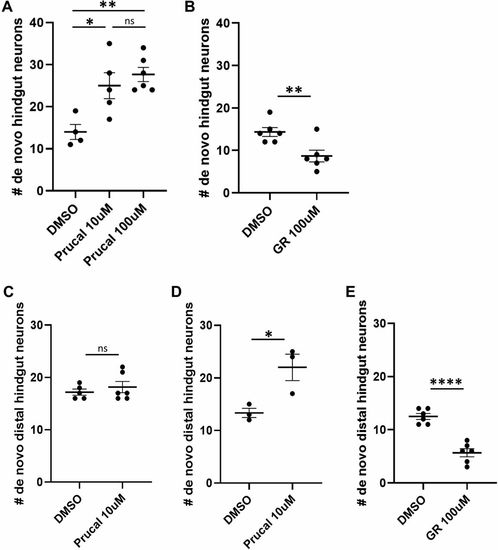
Prucalopride promotes enteric neurogenesis in normal development and injury. (A) After photoconversion of all enteric neurons at 4.5 dpf, cohorts of Phox2b-kaede fish were exposed to 10 µM prucalopride (n=5), 100 µM prucalopride (n=6) or DMSO (n=4) for 12 h and then live imaged at 5 dpf. The mean number of de novo hindgut neurons was significantly higher in fish treated with prucalopride (25, 27.7 and 14, respectively; P=0.019 and P=0.0036, respectively). (B) Under a similar design as A, fish were instead exposed to 100 µM GR 113808 (n=6) versus DMSO (n=6) for 12 h and then re-imaged at 5 dpf. The mean number of de novo hindgut neurons was significantly lower in fish treated with GR 113808 than in controls (8.67 versus 14.33; P=0.0086). (C) At 4.5 dpf, Phox2b-kaede fish underwent laser ablation of ten distal hindgut enteric neurons and then photoconversion of all enteric neurons. Cohorts were exposed to 10 µM prucalopride (n=6) or DMSO (n=5) for 12 h and then live imaged at 5 dpf. There was no difference in de novo distal hindgut neurons between these two groups. (D) Under a similar experimental design as C, fish were instead exposed to 10 µM prucalopride (n=3) or DMSO (n=3) at 3.5 dpf for 12 h, and then underwent cell ablation and photoconversion at 4.5 dpf. At 5 dpf, live imaging revealed significantly more distal hindgut neurons in fish pre-treated with prucalopride (22 versus 13.3; P=0.031). (E) Fish were exposed to 100 µM GR 113808 (n=6) or DMSO (n=6) at 3.5 dpf for 12 h, and then underwent cell ablation and photoconversion at 4.5 dpf. At 5 dpf, live imaging revealed significantly fewer distal hindgut neurons in fish pre-treated with GR 113808 than in controls (5.67 versus 12.50; P<0.0001). Data are mean±s.e.m. *P<0.05, **P<0.01,****P<0.0001. ns, not significant. |