- Title
-
Otoferlin Depletion Results in Abnormal Synaptic Ribbons and Altered Intracellular Calcium Levels in Zebrafish
- Authors
- Manchanda, A., Chatterjee, P., Bonventre, J.A., Haggard, D.E., Kindt, K.S., Tanguay, R.L., Johnson, C.P.
- Source
- Full text @ Sci. Rep.
|
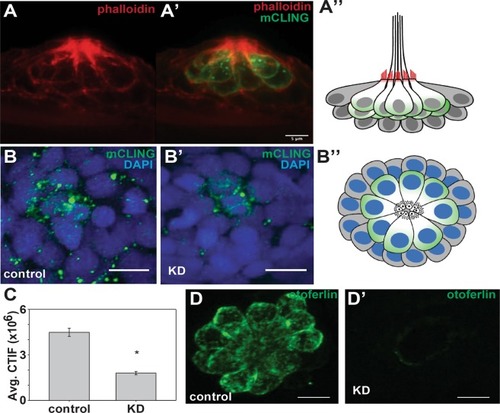
Depletion of otoferlin results in reduced hair cell vesicle recycling. ( |
|
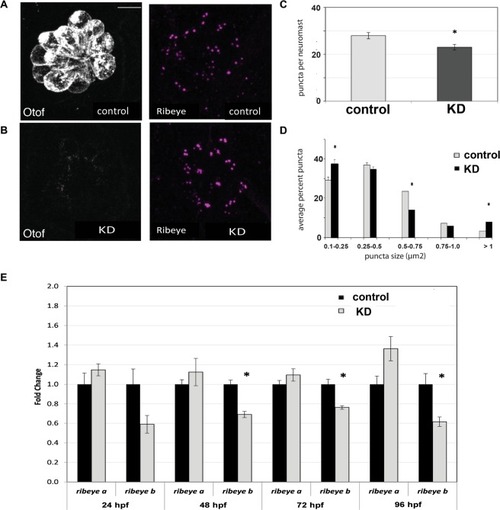
Effects of otoferlin depletion on synaptic morphology in posterior lateral line neuromasts of 96 hpf zebrafish larvae. ( |
|
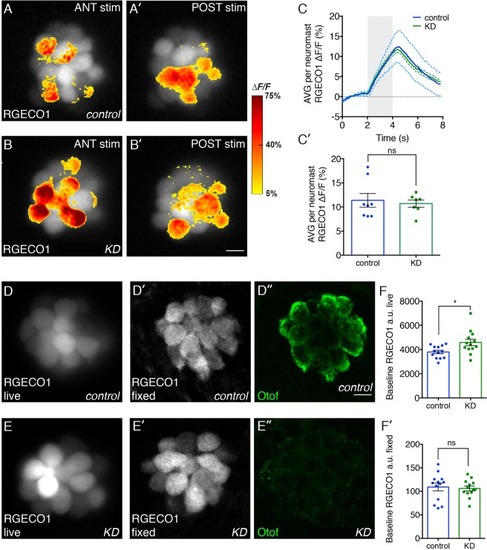
Effects of otoferlin depletion on intracellular calcium in posterior lateral line neuromasts. Representative evoked R-GECO1 calcium signals in negative control injected ( |
|
RNA sequencing and RT-qPCR for differentially expressed genes in otoferlin depleted larvae. ( |