- Title
-
Zika virus enhances monocyte adhesion and transmigration favoring viral dissemination to neural cells
- Authors
- Ayala-Nunez, N.V., Follain, G., Delalande, F., Hirschler, A., Partiot, E., Hale, G.L., Bollweg, B.C., Roels, J., Chazal, M., Bakoa, F., Carocci, M., Bourdoulous, S., Faklaris, O., Zaki, S.R., Eckly, A., Uring-Lambert, B., Doussau, F., Cianferani, S., Carapito, C., Jacobs, F.M.J., Jouvenet, N., Goetz, J.G., Gaudin, R.
- Source
- Full text @ Nat. Commun.
|
Monocyte-derived cells are infected by ZIKV in a human fetus with microcephaly. |
|
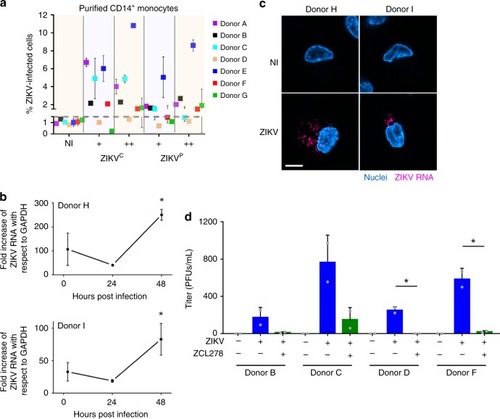
Human primary monocytes are productively infected by ZIKV. |
|
ZIKV-infected monocytes promote viral dissemination and tissue damage to cerebral organoids. |
|
ZIKV-infected monocytes exhibit higher transmigration properties. |
|
ZIKV-exposed monocytes exhibit higher adhesion properties. |
|
ZIKV induces a spread-out morphology of monocytes. |
|
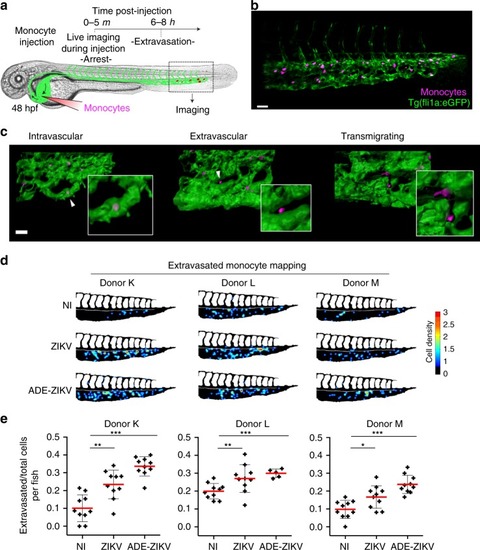
ZIKV enhances monocyte transmigration in zebrafish embryos. |
|
ZIKV favors monocyte arrest in zebrafish vessels, but does not affect their arresting time. Human primary monocytes from three donors were treated and injected as in Fig. |
|
Transmigrated ZIKV-infected monocytes promote viral dissemination to cerebral organoids. |