- Title
-
Aspergillus fumigatus establishes infection in zebrafish by germination of phagocytized conidia, while Aspergillus niger relies on extracellular germination
- Authors
- Koch, B.E.V., Hajdamowicz, N.H., Lagendijk, E., Ram, A.F.J., Meijer, A.H.
- Source
- Full text @ Sci. Rep.
|
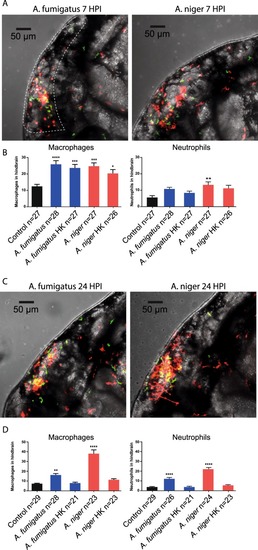
Differences in phagocytic efficiency of |
|
Quantification of leukocyte migration to the hindbrain at early and later stages of infection. ( |
|
Confocal microscopy captures different infectious development of |
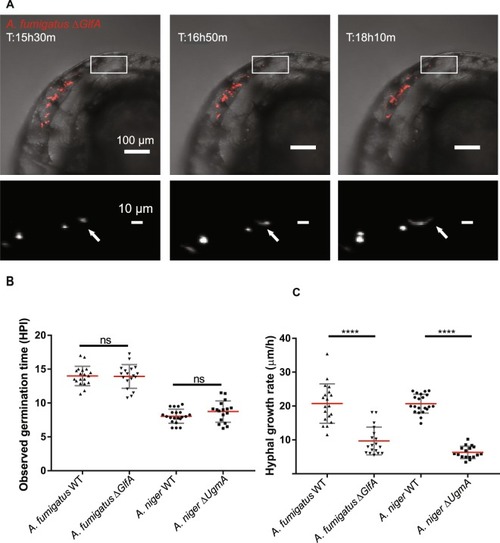
|
Timelapse microscopy based |