- Title
-
Parallels between vertebrate cardiac and cutaneous wound healing and regeneration
- Authors
- Richardson, R.J.
- Source
- Full text @ NPJ Regen Med
|
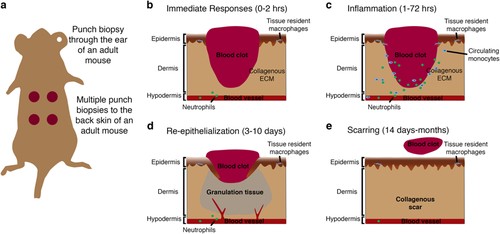
Cutaneous wound healing in adult mouse. a Schematic showing method of inducing several (usually 2–4) punch biopsy full-thickness skin wounds to the back skin of a mouse. b–e Schematics describing the four main stages of cutaneous healing in adult mouse generally defined as: immediate responses including blood clot formation and neutrophil recruitment (0–2 h; b); inflammation involving neutrophil and monocyte recruitment from the peripheral circulation and activation of tissue-resident cells (1–72 h; c); re-epithelialization where keratinocytes proliferate and migrate to re-cover the wound, also coinciding with fibrotic granulation tissue formation, collagen deposition and angiogenic sprouting (3–10 days; d); and finally, the contraction of the wound by myofibroblasts, wound closure, resolution of inflammation and scar remodelling (14 days–months; e) |
|
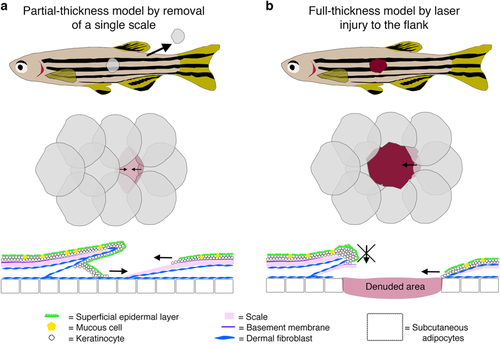
Cutaneous wound healing models in adult zebrafish. a Schematic diagrams describing a single scale removal model of partial-thickness cutaneous wounding in adult zebrafish. b Schematics describing a full-thickness wounding model in adult zebrafish induced using a dermatology laser as previously described.6 In both cases, diagrams at the level of the whole fish (top), flank scales (middle) and cross-section of an individual scale (bottom) are shown. Images were adapted with permission from Richardson et al.7 |
|
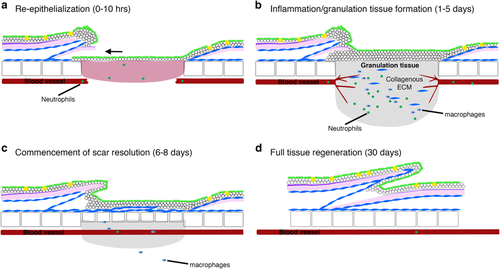
Stages of cutaneous wound healing in adult zebrafish. a Schematic diagrams describing the four main stages of cutaneous wound healing in adult zebrafish: the process of re-epithelisation is extremely rapid and completed within 10 h, preceding almost all other cellular responses; b once the wound is re-covered, neutrophils and macrophages are recruited, a granulation tissue is formed, neo-vascularisation occurs and collagen is deposited beneath the wound; c by 6 days post injury, the granulation tissue and inflammatory responses are reduced and dermal thickenings are starting to reconstitute lost scales; d by approximately 30 days after wounding, the tissue is completely regenerated |
|
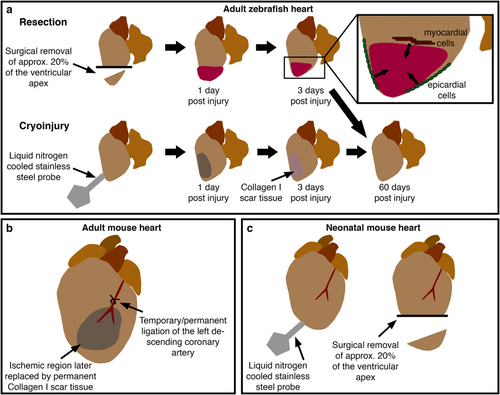
Models of cardiac damage in adult and neonatal vertebrates. a Current methods to induce cardiac damage in adult zebrafish include resection, involving the surgical removal of around 20% of the apex of the ventricle, and cryoinjury where a liquid nitrogen-cooled probe is pressed onto the surface of the ventricle, resulting in cell death and transient scarring. These injury models elicit similar modes of healing involving contributions from de-differentiated epicardial and myocardial cells (inset). b In adult rodents, cardiac injury is usually induced by ligation of the left descending coronary artery. Temporary ligation allows investigation of the effects of reperfusion injury. c Recent reports have described similar injury models to adult zebrafish in neonatal mice eliciting similar cellular responses |