- Title
-
Reduced expression of the Nodal coreceptor Oep causes loss of mesendodermal competence in zebrafish
- Authors
- Vopalensky, P., Pralow, S., Vastenhouw, N.L.
- Source
- Full text @ Development
|
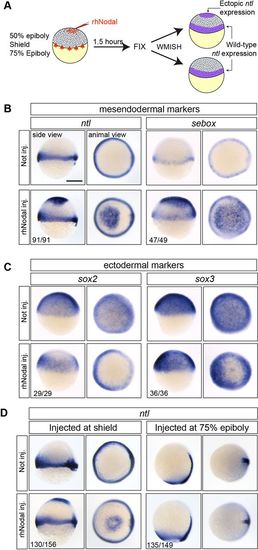
Responsiveness to Nodal is lost between shield stage and 75% epiboly. (A) Experimental setup. WMISH, whole-mount in situ hybridization. (B,C) rhNodal induces ectopic expression of mesendodermal markers (ntl and sebox) (B) and inhibits the expression of ectodermal markers (sox2 and sox3) (C) during zebrafish embryogenesis. (D) rhNodal ectopically induces ntl within the animal cap region at shield stage but not at 75% epiboly. The number of embryos with the indicated expression pattern among the total examined in two (sebox, sox2, sox3) or four (ntl) biological replicates is shown. All side views are shown with dorsal towards the right. Scale bar: 250 µm. |
|
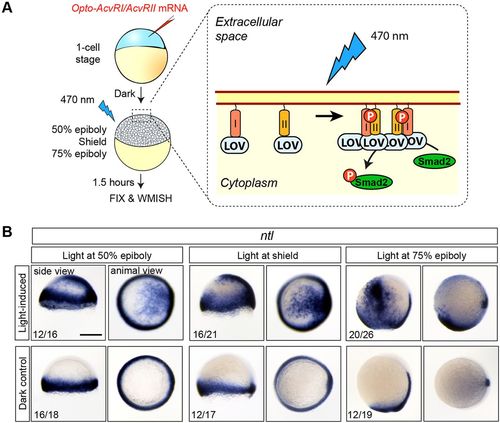
Loss of Nodal competence at 75% epiboly is caused by changes in the extracellular part of the pathway. (A) Experimental setup. I and II, Activin A receptor type I and II intracellular domains; LOV, light-oxygen-voltage domain. (B) Photoactivation of OptoAcvRs results in ectopic ntl expression at all stages examined. Ectopic ntl induction was not observed in control embryos that were kept in the dark. The number of embryos with the indicated expression pattern among the total number examined in two biological replicates is shown. All side views are shown with dorsal towards the right. Scale bar: 250 µm. |
|
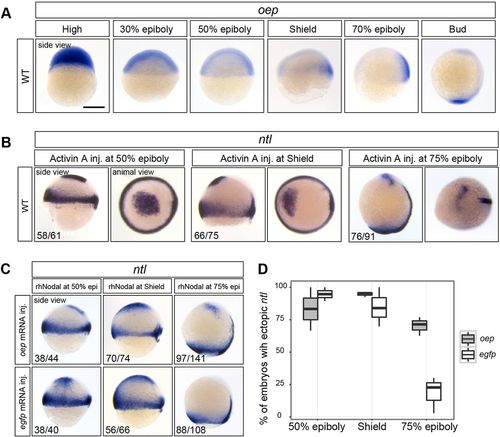
Increasing the level of oep mRNA prolongs the window of responsiveness to Nodal. (A) Time series of oep expression. (B) Intercellular injection of Activin A induces ectopic ntl expression in the animal cap at 50%, shield and 75% epiboly. (C) In oep-injected embryos, but not egfp-injected control embryos, rhNodal is able to induce ectopic ntl at 75% epiboly. (D) Box plot showing the fraction of embryos with ectopic ntl expression (based on data shown in C). The number of embryos with the indicated expression among the total number examined in experiments performed in duplicate (B) or triplicate (C) is indicated. All side views are shown with dorsal towards the right. Scale bar: 250 µm. |
|
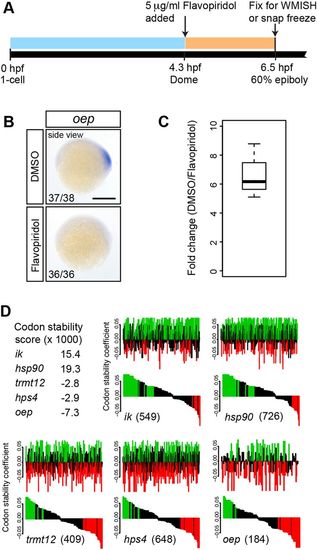
Maternally loaded oep mRNA is globally degraded, at least partially due to the abundance of destabilizing codons. (A) Experimental setup. (B) Embryos treated with flavopiridol lack oep expression at 60% epiboly. The number of embryos with the indicated expression pattern among the total number examined in three biological replicates is shown. All side views are shown with dorsal towards the right. Scale bar: 250 µm. (C) RT-qPCR analysis of nascent oep transcript in control versus flavopiridol-treated embryos. Data are normalized to eif4g2a. The experiment was performed in biological triplicate. (D) oep coding sequence is highly enriched for destabilizing codons. The codon stability score of oep is lower than that of highly stable (ik and hsp90) and even highly unstable (trmt12 and hps4) mRNAs as identified by Bazzini et al. (2016). The distribution of stabilizing (green) and destabilizing (red) codons along the coding sequence is shown (top), along with the same data sorted by codon stability score (bottom). The number of codons in the coding sequence is indicated in parentheses for each gene. See the supplementary Materials and Methods for further details. |
|
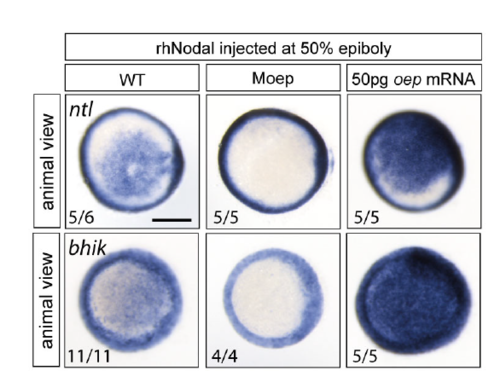
The effect of intercellular injection of rhNodal on target gene expression is mediated by Nodal signaling. Intercellular injection of rhNodal results in the ectopic expression of Nodal target genes ntl and bhik in wildtype embryos, but not in maternal oep (Moep) mutants. Elevated levels of oep result in higher levels of ntl and bhik expression upon intercellular injection of rhNodal. The number of embryos with the indicated expression pattern per total number of embryos examined in one biological replicate is shown. Scale bar 250 μm. |
|
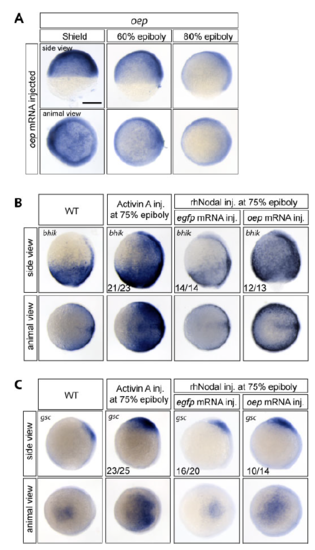
Oep overexpression prolongs the window of induction of Nodal target genes bhik and gsc in prospective ectoderm. A) Injection of 50pg oep mRNA at the 1-cell stage extends the expression level of oep to late gastrulation stages (compare to Fig. 3A). (B,C) Both bhik (B) and gsc (C) show the same induction response as ntl. At 75% epiboly, both genes can be ectopically induced by Activin A injection but not by rhNodal injection into egfp mRNA-injected embryos. When oep is overexpressed, rhNodal injection also results in ectopic expression of target genes. The number of embryos with the indicated expression pattern per total number of embryos examined in one biological replicate is shown. Scale bar 250 μm. |
|
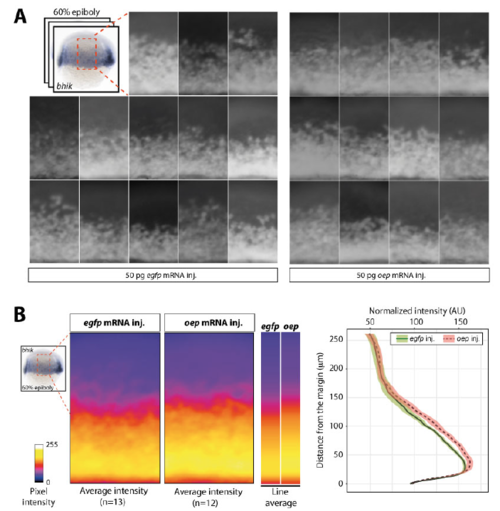
Overexpression of oep results in expansion of bhik expression domain towards the animal pole. A) Lateral view of the expression of bhik in individual 60% epiboly embryos injected with egfp or oep mRNA. B) Signal intensity of the lateral area averaged over multiple embryos is shown in the heatmap panels. Average intensity of bhik staining profiles along the animal-vegetal axis for both treatments are shown in heatmap bars. Corresponding values are plotted in the graph. Colored envelopes of the curves represent the standard error of the mean, based on 12 (oep) and 13 (egfp) embryos. bhik expression is similar in oep- and egfp-injected most animally and vegetally, but in oep-injected embryos, the average intensity is higher in the mesendoderm area, and the expression domain is extended towards the animal pole. Shown is a representative example of three biological replicates. Although this expansion of the reach of Nodal signaling does not impede development ((Zhang et al., 1998), this study), the decrease in oep expression might be relevant under altered environmental conditions. |