- Title
-
Live-cell imaging: new avenues to investigate retinal regeneration
- Authors
- Lahne, M., Hyde, D.R.
- Source
- Full text @ Neural Regen Res
|
Schematic of the time course of the regenerating zebrafish retina. Simplified diagram of a healthy retina (a). Exposure of zebrafish to constant intense light induces photoreceptor cell death (b). The dying photoreceptors are engulfed by microglia (pink, a–e, k–n) or Müller glia (green, b, c, k, l). At subsequent time points, a subset of Müller glia re-enters the cell cycle, expressing the proliferating cell nuclear antigen, PCNA (c). Proliferating Müller glia nuclei undergo interkinetic nuclear migration (INM) to the outer nuclear layer (ONL) to divide, producing a neuronal progenitor cell (NPC) and a Müller glia (d). Both nuclei return to the inner nuclear layer (INL) where the Müller glia exits the cell cycle (checkered nucleus), while the NPC continues to proliferate (e). NPCs also undergo INM (f, black and red arrows represent apical and basal migration, respectively), producing clusters of NPCs that increase expression of commitment factors that drive NPCs towards the photoreceptor lineage in the light-damaged retina (g). Committed NPCs migrate to the ONL (h), differentiating into the lost neurons to repair the damaged retina (i, checkered nuclei). In addition, a few inner retinal neurons are also produced during the regeneration time course (i, checkered nuclei in INL and ganglion cell layer (GCL)). A subregion of the ONL was magnified to display events in the ONL in detail (j–q). AC: Amacrine cell; MG: Müller glia. |
|
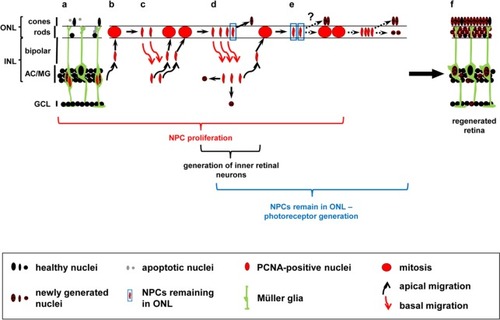
Hypothetical model of NPC-mediated generation of neuronal subtypes in the regenerating zebrafish retina. Diagram of a light-damaged retina after the initial Müller glia cell division that produced a cell cycle exiting Müller glia (a, checkered nucleus) and an NPC that continues to proliferate (a, red nucleus). NPCs undergo several rounds of cell divisions (b–e), migrating repetitively in phase with the cell cycle between the INL where they replicate their DNA and the ONL where mitosis occurs (b–d, round red nucleus). Black and red arrows indicate apical and basal nuclear migration, respectively. Mitosis potentially produces cells with neurogenic and proliferative fates allowing a subset of cells to sequentially exit the cell cycle (d, e), thereby generating cells that differentiate into the different retinal subtypes. A subset of ONL cell divisions produce nuclei that remain in the ONL (d, e, blue boxed nuclei). These ONL remaining cells could either directly exit the cell cycle and differentiate into the lost photoreceptors (d, e; checkered ONL based nuclei) or undergo an additional cell division before giving rise to photoreceptors (e) resulting in the regeneration of the damaged retina (f). This model is based on the following research: 1) Müller glia divide asymmetrically yielding a cell-cycle exiting Müller glia and an NPC that continues proliferating (Nagashima et al., 2013), 2) only 61 percent of nuclei return from the ONL to the INL after cell division (Lahne et al., 2015), and 3) both photoreceptors and inner retinal neurons are produced following light damage (Vihtelic and Hyde, 2000; Lahne et al., 2015; Powell et al., 2016). The exact sequence of events leading to the differentiation of the different neuronal subtypes in the regenerating zebrafish retina is currently unknown. AC: Amacrine cell; GCL: ganglion cell layer; INL: inner nuclear layer; MG: Müller glia; NPC: neuronal progenitor cell; ONL: outer nuclear layer; PCNA: proliferating cell nuclear antigen. |