- Title
-
Harmless effects of argon plasma on caudal fin regeneration and embryogenesis of zebrafish: novel biological approaches for safe medical applications of bioplasma
- Authors
- Nam, M.K., Kim, G.Y., Yun, S.E., Jang, J.Y., Kim, Y.H., Choi, E.H., Kang, S., Rhim, H.
- Source
- Full text @ Exp. Mol. Med.
|
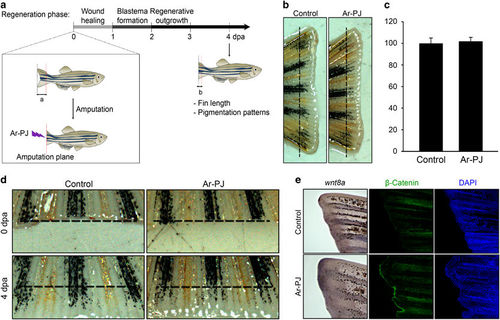
Biosafety of Ar-PJ in living tissues—experimental validation of the regenerative capacity of the zebrafish caudal fin as a novel biosafety assessment system. (a) A schematic timeline of caudal fin regeneration and outline for regeneration experiments. Caudal fins of the zebrafish (aged 3 months) were amputated with a surgical blade and treated with Ar-PJ for 30 s, and the zebrafish were incubated at 33 °C for the indicated times. ‘a’ and ‘b’ denote the lengths from the amputation plane to the distal tip of original fin and of the newly forming fin after amputation, respectively. (b) Regrowth rate of the amputated-zebrafish caudal fins, which is a measurable indicator for assessing Ar-PJ biosafety in in vivo tissue networks. Fins processed via the experiments outlined in (a) were photographed at 4 dpa using a MZ FLIII stereomicroscope. (c) Quantification of the regrowth rate. Regrowth was calculated as the ratio of ‘b’ relative to ‘a’. The ratio of the Ar-PJ treated sample was divided by that of the untreated control, and the values are expressed as a percentage of the control. Data are presented as the mean values±s.e.m. (n=6). (d) Expansion patterns of pigment cells in the regenerating fins, a visual indicator of the biosafety of Ar-PJ in cellular networks. Melanocytes (black) and xanthophore cells (yellow) are arranged according to their cell-to-cell interactions. (e) Detection of the regeneration-associated genes, wnt8 and β-Catenin, in the regenerating fins, molecular indicators of the biosafety of Ar-PJ in living organisms. WISH was performed on zebrafish fins at 4 dpa with a wnt8a probe. Violet color indicates a positive signal of the wnt8a mRNA. Immunofluorescence antibody assay (IFA) was performed on zebrafish fins at 2 dpa with the anti-active-β-Catenin antibody (green), and nuclei were counterstained with DAPI (blue). |
|
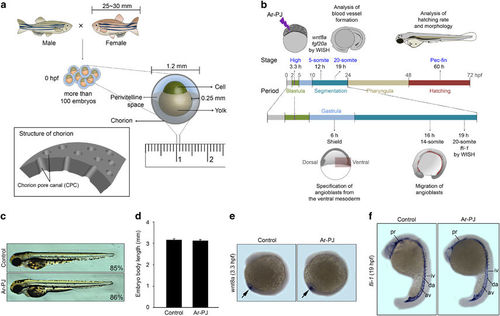
Biosafety of Ar-PJ with respect to the biogenesis of living organisms—experimental validations of embryogenesis as a novel biosafety assessment system. (a) Schematic diagrams of zebrafish fertilization and the structure of an embryo at 0 hpf. (b) A schematic diagram of experimental designs for assessing zebrafish embryogenesis. (c) Evaluation of the completion of embryogenesis in response to Ar-PJ, a visual indicator of Ar-PJ biosafety. Hatched embryos were counted at 60 hpf, and the hatching rate was determined by calculating the hatching percentage (H%) for each sample: H%=(the number of hatched embryos/the total number of embryos) × 100. Lateral view, anterior to the top. (d) Quantification of body length—a measurable indicator of Ar-PJ biosafety assessment. The lateral length of embryos at 60 hpf was measured, and values are presented as the means±s.e.m. (control n=7, Ar-PJ n=12). (e) Evaluation of Ar-PJ biosafety at the molecular level. Live zebrafish embryos at the blastula stage (3.3 hpf) were treated for 30 s with Ar-PJ. WISH was performed with a wnt8a antisense probe on segmentation-period embryos (12 hpf, 5-somite stage). (f) Evaluation of blood vessel morphogenesis, an embryonic visual indicator for Ar-PJ biosafety assessment. WISH was performed with a fli antisense probe on segmentation-period embryos (19 hpf). Vasculogenesis sites are indicated: pharyngeal (pr), dorsal aorta (da), axial vein (av), intersegmental vessels (iv) and intermediate cell mass (icm). |
|
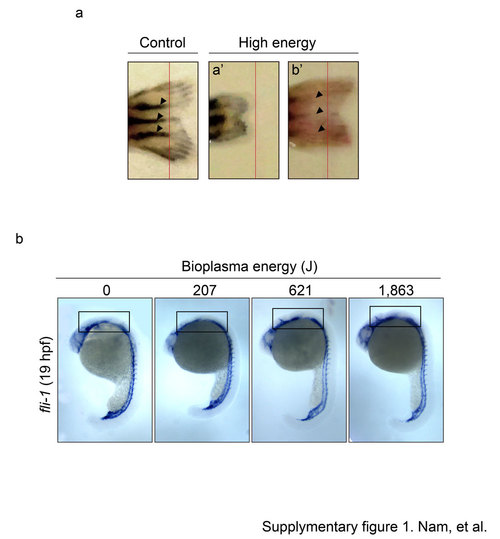
Supplementary Figure 1. Ar-PJ has impaired effects on caudal fin regeneration and embryonic vasculogenesis. a. Regrowth of the amputated-zebrafish caudal fins. Fins were photographed at 20 dpa (day per amputation). Red lines indicate the amputation line. b. Blood vessel morphogenesis with increasing energy of bioplasma. Open boxes indicate the posterior head region. |