- Title
-
Zebrafish zic2 controls formation of periocular neural crest and choroid fissure morphogenesis
- Authors
- Sedykh, I., Yoon, B., Roberson, L., Moskvin, O., Dewey, C.N., Grinblat, Y.
- Source
- Full text @ Dev. Biol.
|
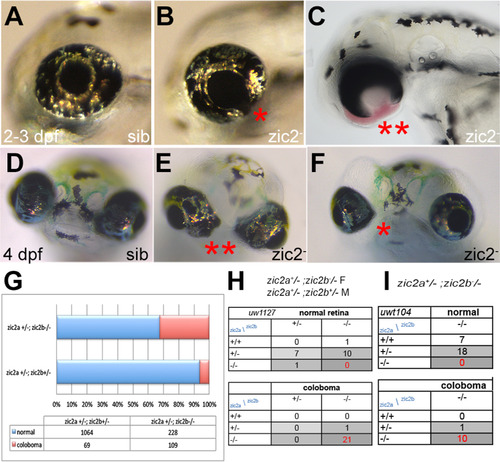
Zebrafish Zic2 is required during retinal morphogenesis. A: normal retinal morphology. B: retina exhibiting mild coloboma (*). C: retina exhibiting severe coloboma with periocular hemorrhaging (**). D: normal retinal morphology. E: bilateral coloboma in a severely affected embryo (**). F: mild, unilateral coloboma in an affected embryo. G: Penetrance and expressivity of coloboma is increased in progeny that lack maternal zic2b, derived from zic2agbt133/+; zic2bt104/zic2bt104 parents, compared to those from double heterozygous (zic2agbt133/+; zic2bt104/+) parents (see Table S1 for details). H, I: Both CRISPR- and TALEN-induced mutant alleles of zic2b are tightly associated with coloboma in MZ-zic2 embryos. Embryos in A-C are at 2–3 dpf, shown in lateral views, anterior to the left. Embryos in D-F are at 4 dpf, shown in anterior views, dorsal at the top. PHENOTYPE:
|
|
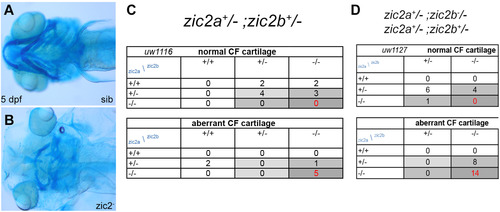
Zebrafish Zic2 is required for craniofacial cartilage development. A: normal neurocranium and branchial arches. B: hypoplastic, disorganized craniofacial cartilages in a zic2 mutant. C: Craniofacial defects are enriched in zic2 mutants derived from double heterozygous parents. D: In embryos that lack maternal zic2b, craniofacial defects are observed in zic2 mutants and in embryos with one wildtype copy of zic2a. Cartilage was visualized by staining with Alcian Blue. Embryos at 5 dpf are shown in ventral views, anterior to the left. |
|
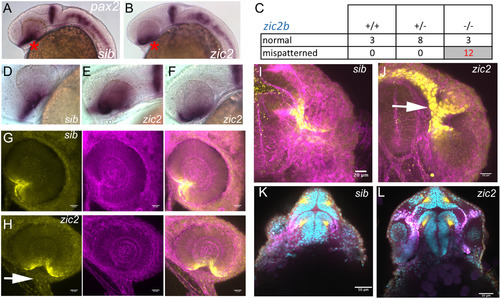
Pax2a expression is aberrant in MZ-zic2 mutants at 1 dpf. pax2a expression at 1 dpf was visualized in embryos derived from zic2agbt133/+; zic2buw1116/+ parents using WISH (A-F) or in progeny of zic2agbt133/+; zic2buwt104 parents using immunohistochemistry (G-L). A: normal pax2a expression in the ventral retina (*). B: mispatterned pax2a expression (*) was observed in 12 out of 103 embryos (12%, 2 expts.). C: Only zic2b homozygous embryos exhibit pax2a mispatterning. zic2a genotype was not tested because PCR genotyping was not robust after WISH. D-F: Embryos with mispatterned pax2a expression also exhibit coloboma, indicative of homozygosity for zic2agbt133. G, H: confocal stacks through representative retina of normal (G) and zic2 mutant (H) retina. I, J: confocal stacks through the ventral aspects of a normal (I) and zic2 mutant (J) diencephalon and retina. Arrowheads in H, J point to the aberrant optic stalk. In G-J, yellow = Pax2a, magenta = F-actin cytoskeleton visualized by phalloidin. K, L: single confocal sections through representative normal (K) and zic2 mutant (L) embryos, imaged ventrally at the level of choroid fissure. magenta = Pax2a; yellow = acetylated tubulin; cyan = nuclei visualized by DAPI. Embryos are shown in lateral views, anterior to the left (A-F) or anterior to the right (G, H); in ventral views with anterior at the top (I-L). |
|
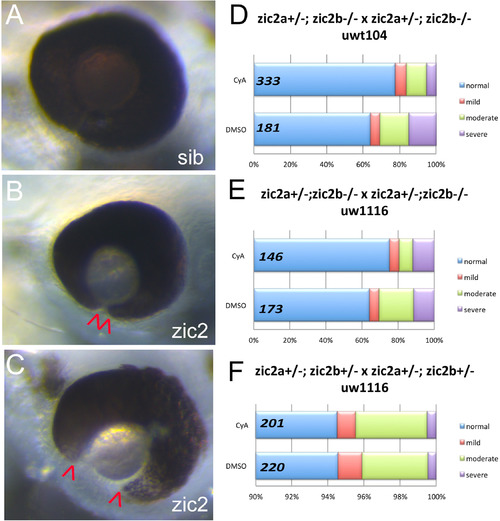
Cyclopamine treatment reduces frequency and severity of coloboma in zic2 mutants. A: normal retinal morphology; B: retina with mild coloboma; C: retina with moderate coloboma. D: Embryos were derived from zic2agbt133/+; zic2b t104 parental crosses and exposed to 3 or 4.5 μM cyclopamine (CyA) from 3 to 5 hpf until 24–26 hpf. In CyA-treated groups (3 expts; Fig. S3A), the proportion of embryos with coloboma was reduced significantly compared to vehicle-treated control siblings (Fisher’s Exact test, P < 0.001). Proportion of severely affected embryos among all embryos with coloboma was also decreased in CyA-treated siblings (Fisher Exact test, p < 0.02). E: Embryos were derived from zic2agbt133/+; zic2b uw1116 parents, and treated starting at 3 hpf with 4.5 μM Cya. CyA-treated groups exhibited reduction in coloboma penetrance (Fisher’s Exact test p < 0.04) compared to vehicle-treated control siblings (2 expts; Fig. S3B). F: Embryos were derived from zic2agbt133/+; zic2b uw1116/+ parents, and treated as in D with 4.5 μM CyA or vehicle starting at 3 hpf. Proportion of embryos with coloboma was not affected by exposure to cyclopamine (2 expts). Embryos with unilateral mild coloboma were scored as “mild”; embryos with bilateral mild coloboma were scored as “moderate”, and embryos with bilateral moderate coloboma were scored as “severe”. |
|
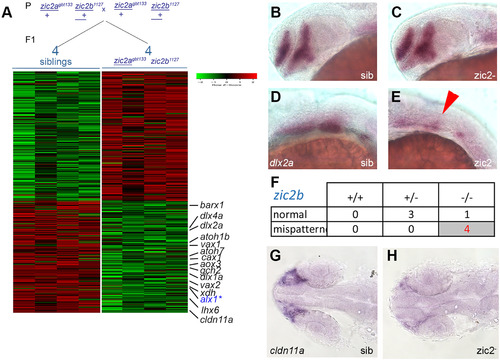
RNA sequencing transcriptome analysis identifies a set of Zic2-dependent targets. A: Embryos derived from zic2agbt133/+; zic2b1127/+ parents were sorted by presence or absence of coloboma into zic2 mutants and sibling groups, respectively. RNA extracted from individual embryos (4 wildtype and 5 with coloboma) was used to prepare cDNA libraries for illumina high-throughput sequencing. Genes with assigned value of False-Discovery Rate below 0.05 were preliminarily selected. The heat-map color represents relative expression levels of differentially expressed genes; 4 out of 5 coloboma-representing libraries are shown to maintain visual balance with the 4 normal sibling samples. B, C: Representative sibling and zic2 mutant embryos derived from zic2agbt133/+;zic2buw1116/+ parents show normal dlx2a expression by WISH in the telencephalon and diencephalon. D: normal dlx2a expression in branchial arch primordia. E: depleted branchial arch dlx2a expression (arrowhead) was observed in 7 out of 127 (6%, 3 expts.) of embryos from this cross. F: Only zic2b- homozygotes exhibited dlx2a reduction in branchial arch primordia. G: normal cldn11a expression adjacent to the optic stalk of embryo with normal retinal morphology. H: depleted cldn11a expression in zic2 mutant with coloboma. Embryos in B – E are shown in lateral views, anterior to the left. Embryos in G and H are shown in dorsal views, anterior to the left. |
|
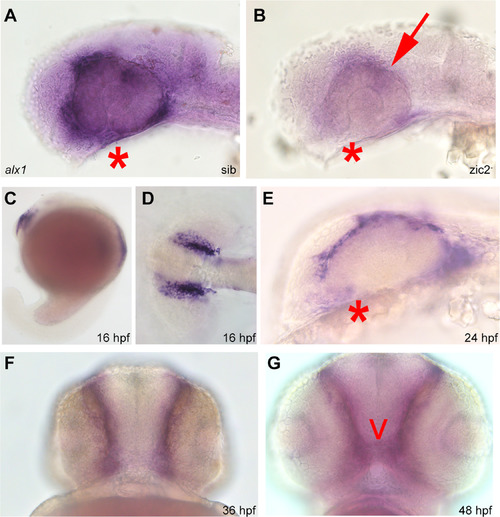
Alx1 is a novel target of zic2 in the periocular neural crest. Embryos derived from zic2agbt133/+; zic2bt104/zic2bt104 parents were stained for alx1 expression by WISH. A: normal expression in periocular mesenchyme of sibling embryo. B: depleted expression in zic2 (arrow) in mutant embryo (39 out of 112 total, 2 expts). C-G: wild type embryos stained for alx1 expression by WISH. C-D: alx1 is expressed in frontonasal neural crest at 16 hpf. E, F: alx1 is expressed in periocular mesenchyme (*) at 24 hpf and 36 hpf. G: alx1 is expressed in the ethmoid plate (arrowhead) at 48 hpf. Embryos in A, B, C, and E are shown in lateral views, anterior to the left. Embryo in D is shown dorsally, anterior to the left. Embryos in F and G are shown in anterior views, ventral at the top. |
|
Frontonasal and pharyngeal neural crest is depleted in MZ-zic2 mutants. A-C: normal crestin expression in frontonasal and pharyngeal neural crest. D-F: depleted crestin expression in 14 of 55 embryos from a zic2agbt133/+; zic2bt104/zic2bt104 incross. Arrows point to periocular neural crest. Arrowhead points to pharyngeal arch expression. Embryos are shown in lateral views, anterior to the left. |
|
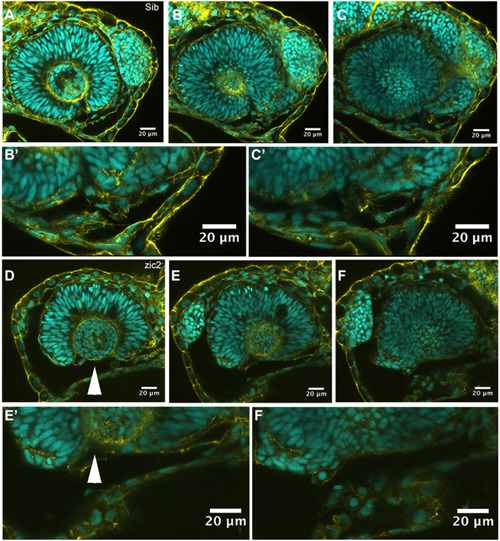
Ventral periocular neural crest is depleted in MZ-zic2 mutants. Single confocal sections through optic cups of embryos derived from zic2agbt133/+; zic2b1116/zic2b1116 parents. A-C’: embryo with normal retinal morphology. D-F’: embryo with coloboma. Embryos were imaged in lateral mounts. Cyan = nuclei visualized by DAPI; yellow=F-actin cytoskeleton visualized by phalloidin. Arrowheads point to aberrant gap in the ventral retina (coloboma). Embryos are shown in lateral views, anterior to the right (A-C) or anterior to the left (E-G). B’, C’, E’, F’ are enlarged from B, C, E and F, respectively. |
|
Generation and characterization of mutant alleles at zic2a and zic2b loci. A: zic2agbt133 was identified in an insertional mutagenesis screen (Clark et al., 2011). This insertion in the first coding exon of zic2a, near the N-terminus of the predicted open reading frame of zic2a. This allele is predicted to be a functional null. Tracks from individual embryos deep-sequenced on the Illumina platform show that the insertional allele inhibits transcription of the first coding exon of zic2a. B: CRISPR and TALEN nucleases were designed to target different sites in the first coding exon of zic2b.Two mutant alleles of zic2b were generated using CRISPR mutagenesis (uw1127 and uw1116). A third allele of zic2b was generated using TALEN mutagenesis (uwt104). Uwt104 and uw1116 cause frameshifts; uw1127 is an in-frame insertion that contains a translational termination codon. All three alleles encode truncated proteins that do not contain the ultra-conserved zinc binding domains. C: normal 5 dpf sibling and D: zic2 mutant with cerebral edema (arrow) from a zic2 heterozygous carrier incross. PHENOTYPE:
|
|
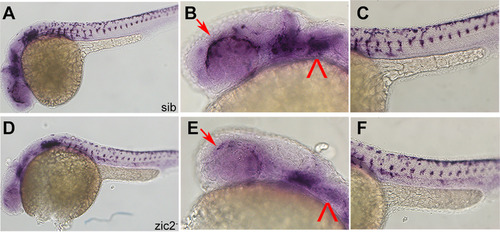
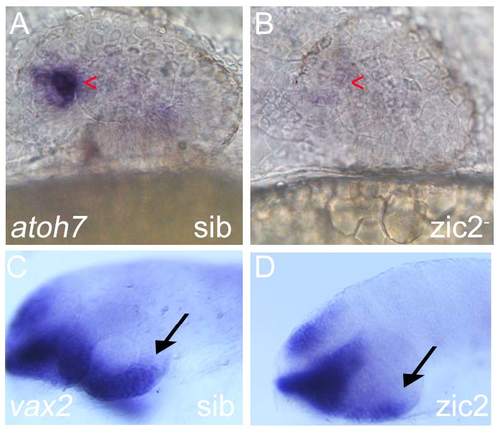
Aberrant expression of Atoh7 and vax2a in MZ-zic2 mutants. Embryos derived from zic2agbt133/+; zic2bt104/zic2bt104 parents were stained for alx1 expression by WISH. A: normal atoh7 expression in retinal precursor cells (arrowhead); B: depleted atoh7 expression in 8 out of 33 embryos (24.2%). C: normal vax2a expression in choroid fissure (arrow); D: depleted vax2a expression. Embryos are shown in lateral views, anterior to the left. |
Reprinted from Developmental Biology, 429(1), Sedykh, I., Yoon, B., Roberson, L., Moskvin, O., Dewey, C.N., Grinblat, Y., Zebrafish zic2 controls formation of periocular neural crest and choroid fissure morphogenesis, 92-104, Copyright (2017) with permission from Elsevier. Full text @ Dev. Biol.