- Title
-
Dynamic regulation of VEGF-inducible genes by an ERK/ERG/p300 transcriptional network
- Authors
- Fish, J.E., Cantu Gutierrez, M., Dang, L.T., Khyzha, N., Chen, Z., Veitch, S., Cheng, H.S., Khor, M., Antounians, L., Njock, M.S., Boudreau, E., Herman, A.M., Rhyner, A.M., Ruiz, O.E., Eisenhoffer, G.T., Medina-Rivera, A., Wilson, M.D., Wythe, J.D.
- Source
- Full text @ Development
|
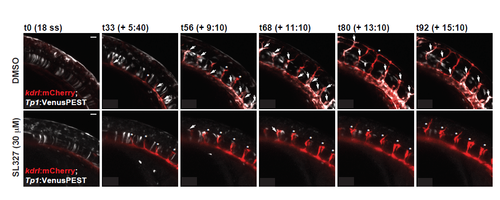
Active MAPK/ERK signaling regulates sprouting angiogenesis in zebrafish. (A) pERK staining (white) in embryos treated with vehicle (i.e. DMSO), MEK (i.e. SL327) or VEGFR2 (i.e. SU5416) inhibitors [treated from 18-20 hours post-fertilization (hpf) to ∼24 hpf]. See Fig. S2A for additional images and quantification. Yellow arrows indicate pERK-positive sprouting endothelial cells. (B) Inhibition of MEK activity by SL327 inhibits ISV sprout length. Inhibition of VEGFR2 signaling with SU5416 is included as a positive control. Quantification of ISV length at 28 hpf is shown. (C) dll4 expression in SL327-treated embryos at 28 hpf (treatment initiated at 18-20 hpf) as assessed by qRT-PCR (n=6 individual embryos). (D) Notch activity is reduced in the vasculature of SL327-treated Tg(kdrl:mCherry); Tg(Tp1bglob:Venus-PEST) embryos. Still images from time-lapse microscopy of a representative experiment are shown. Arrows indicate Notch signaling-positive ISVs, arrowheads indicate Notch signaling-positive endothelial cells in the dorsal aorta, asterisks indicate Notch-negative ISVs. See Fig. S3 (for additional still images) and Movies 1 and 2. (E) Similar experiment to that shown in D, but with Tg(kdrl:mCherry); Tg(Tp1bglob:EGFP) embryos. Scale bars: 50 μm (A,B,E); 20 μm (D). |
|
VEGF/MAPK signaling stimulates ERG transcriptional activity to induce DLL4 expression. (A) VEGF induction of DLL4 transcription (as assessed by qRT-PCR measurement of DLL4 pre-mRNA) and mature DLL4 mRNA expression in HUVECs requires ERG (n=4). (B) VEGF induction of DLL4 protein expression requires ERG. Representative experiment of three. (C) Induction of DLL4 transcription by PMA, an activator of MAPK/ERK signaling, requires ERG (n=4). (D) ETS activity (as assessed by activity of an 8× concatamer of an ETS element driving luciferase expression) in BAECs is suppressed by MEK or PKC inhibition. Triplicate determinations from a representative experiment of three. (E) ERG is phosphorylated at S215 in response to VEGF stimulation (15-30′) in HUVECs. Representative experiment of two. (F) VEGF-induced phosphorylation of ERG requires MEK activity. Representative experiment of two. (G) ERG was knocked down using siRNAs directed to the 3′ UTR, followed by overexpression of Flag-tagged wild-type (WT) or mutant (S215A or S96A;S215A;S276A, indicated as 3xS→A) ERG. ERG western blot indicates restoration of expression using electroporated constructs (a representative experiment of three is shown). DLL4 expression as assessed by qRT-PCR of pre-mRNA after 1 h of VEGF treatment (n=3). (H) Representative images of transplanted cells from Tg(fli1a:nls-GFP) embryos injected with wild-type or mutant ERG mRNA into Tg(kdrl:mCherry) embryos. Arrows and asterisks indicate endothelial cells that are donor derived. Quantification of cellular position is shown below (n=248 cells from 11 embryos for wild type and n=340 cells from 14 embryos for mutant). Scale bar: 50 µm. DA, dorsal aorta (asterisks); DLAV, dorsal longitudinal anastomotic vessel (arrowheads); ISV, intersomitic vessel (arrows); NS, non-stimulated; PCV, posterior cardinal vein. |
|
p300 is dynamically recruited to the DLL4 enhancer and regulates sprouting angiogenesis. (A) Recruitment of p300 to the DLL4 enhancer located in intron 3 15-30′ after VEGF stimulation, as assessed by ChIP (n=5 for ERG and p300 ChIP, n=3 for H3K27ac ChIP). (B) p300 recruitment in response to VEGF stimulation requires ERG. Shown is a representative experiment of two with triplicate determinations. The extent of ERG knockdown as assessed by western blot is shown to the right. (C) Endogenous p300 and ERG physically interact by co-immunoprecipitation in HUVECs stimulated with VEGF. Shown is a representative experiment of three. (D) Exogenous Myc-p300 interacts with wild-type Flag-ERG in BAECs, but does not interact with phospho-mutant (S96A;S215A;S276A) Flag-ERG by co-immunoprecipitation. Shown is a representative experiment of three. (E) p300 activity is required for DLL4 mRNA induction in HUVECs in response to VEGF stimulation (n=5). c646 is a potent inhibitor of p300/CBP activity. (F) Inhibition of p300 activity suppresses ISV elongation in zebrafish. Quantification is shown to the right. Note: Quantification of the DMSO control is the same as that shown in Fig. 2B, as both inhibitors were used in the same experiment. Scale bars: 50 μm. NC, negative control (V5 antibody). |
|
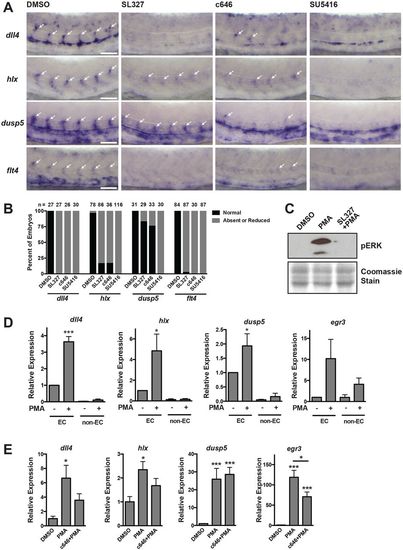
Regulation of VEGF/ERK/ERG/p300-dependent genes in vivo. (A) In situ hybridization using probes for dll4, hlx1, dusp5 and flt4 at 26 hpf. Embryos were treated with inhibitors of MEK (SL327), p300/CBP (c646) or VEGF (SU5416) starting at the 20-somite stage. Expression of each of these genes is MEK, p300 and VEGF dependent. Arrows indicate ISVs expressing the indicated genes. Representative images are shown. (B) Quantification of in situ hybridization experiments. The number of embryos analyzed is indicated. (C) pERK western blot in embryos pre-treated with SL327 for 1 h, followed by addition of PMA for 2 h. Coomassie staining was used to assess loading. Representative experiment of two. (D) qRT-PCR analysis of endothelial or non-endothelial cells isolated from kdrl:GFP embryos exposed to PMA for 2 h (starting at 24 hpf). All of these genes are induced in the endothelium in response to ectopic MEK activation (n=3). (E) qRT-PCR of whole individual embryos that were exposed to DMSO or c646 for 1 h, prior to stimulation with PMA for 2 h at 24 hpf. The induction of dll4, hlx and egr3 by PMA is p300 dependent (n=6). |
|
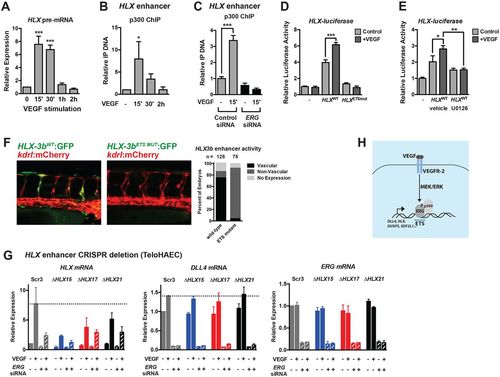
A conserved enhancer upstream of HLX is regulated by ETS factors and is required for VEGF induction. (A) Transcription of HLX (as assessed by qRT-PCR of HLX pre-mRNA) reveals dynamic transcription, peaking at 15-30′ post-VEGF stimulation in HUVECs (n=3). (B) p300 is transiently recruited to a putative HLX enhancer element during VEGF stimulation, as assessed by ChIP assay (n=3). (C) p300 ChIP was performed in control and ERG knockdown cells. Shown are triplicate measures of a representative experiment of two. (D) Luciferase analysis of the HLX enhancer (HLX-3a), demonstrating that it is regulated by VEGF and ETS factors. ETS-binding sites were mutated in the HLX enhancer (HLXETSmut, see Materials and Methods). A representative experiment (of three) with triplicate determinations is shown. (E) Luciferase analysis of the HLX enhancer (HLX-3a), demonstrating that it is regulated by MAPK/ERK activity. A representative experiment (of two) with triplicate determinations is shown. (F) The human HLX enhancer (HLX-3b) is functional in ISVs in zebrafish during sprouting angiogenesis. Activity is lost when the ETS sites in the enhancer are mutated. Shown are representative images of embryos at 42 hpf. Quantification of enhancer activity is shown to the right. (G) CRISPR/Cas9-mediated deletion of a highly conserved enhancer upstream of HLX inhibits VEGF-mediated induction. Shown are a clonal scrambled-control line (Scr3) and three heterozygous deletion lines (ΔHLX15, ΔHLX17, ΔHLX21). Induction of DLL4 is included as a control. Knockdown of ERG affects the induction of HLX in the control line to a greater extent than in the deletion lines. n=2. (H) Schematic of the VEGF/MEK/ERK/ERG/p300 transcriptional pathway identified in this study. |
|
Characterization of sprouting defects in embryos treated with MEK inhibitor (SL327). A) pERK is enriched in tip cells during ISV formation in zebrafish and this is dependent on MEK signaling as it is negated in MEK-inhibitor (SL327) treated embryos (treated from 18-20 hpf to ~24 hpf). Quantification of the number of ISVs that stained positive for pERK is indicated. 24 hpf. Scale bar = 50 μm. B) The number of endothelial cells per intersomitic vessel was quantified in Tg(kdrl:mCherry;fli1a:nls-EGFP) embryos exposed to DMSO or SL327 (30 μM). The number of mCherry/EGFP double-positive cells was counted per intersomitic vessel and averaged per embryo. The number of embryos quantified is indicated. C) Transmitted light images of DMSO, SL327 (30 μM), SU5416 (5 μM) and c646 (3 μM) treated embryos revealing normal overall development and lack of tissue necrosis or developmental delay. |
|
Notch induction in the vasculature is compromised in embryos treated with MEK inhibitor (SL327). Notch activity is reduced in the vasculature of SL327-treated Tg(kdrl:mCherry); Tg(Tp1bglob:Venus-PEST) embryos. Still images from time-lapse microscopy of a representative experiment are shown. Arrows indicate Notch signaling-positive ISVs, while asterisks indicate Notch-negative ISVs. Scale bar = 20 μm. See Videos S1 and S2. |