- Title
-
Small fluorescence-activating and absorption-shifting tag for tunable protein imaging in vivo
- Authors
- Plamont, M.A., Billon-Denis, E., Maurin, S., Gauron, C., Pimenta, F.M., Specht, C.G., Shi, J., Quérard, J., Pan, B., Rossignol, J., Moncoq, K., Morellet, N., Volovitch, M., Lescop, E., Chen, Y., Triller, A., Vriz, S., Le Saux, T., Jullien, L., Gautier, A.
- Source
- Full text @ Proc. Natl. Acad. Sci. USA
|
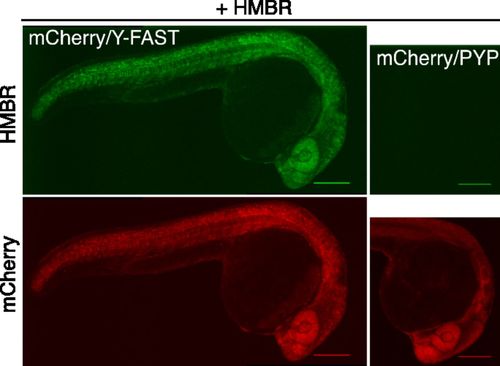
Specific labeling of fusion proteins in vivo. Spinning-disk confocal micrographs of live zebrafish embryos coexpressing mCherry/Y-FAST or mCherry/PYP labeled with 5 μM HMBR at 24 h postfertilization (HMBR channel: Ex/Em 491/525–539 nm; mCherry channel: Ex/Em 561/605–664 nm). (Scale bars, 200 μm.) Side-by-side images were recorded using the same settings. |
|
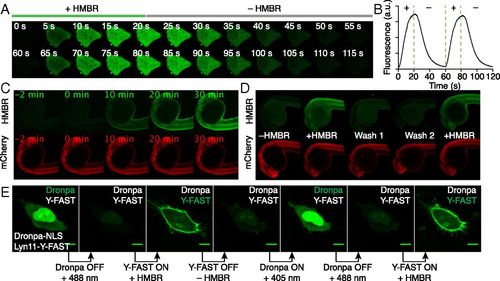
On/off fluorescence switching by iterative labeling/unlabeling. (A and B) HeLa cells expressing mCherry-Y-FAST were grown in a microfluidic channel and repeatedly incubated with HMBR-containing culture medium for 20 s and HMBR-free culture medium for 40 s. A multifunctional fluidic controller enabled several cycles of labeling/unlabeling. HMBR concentration was 5 μM. (A) Confocal time lapse showing two cycles of labeling/unlabeling (Ex/Em 488/493–575 nm). Movie S2 shows 10 cycles of labeling/unlabeling. (B) Temporal evolution of the cell fluorescence upon addition (+) and removal (–) of HMBR. (C) Confocal time lapse showing the labeling kinetics in a zebrafish embryo expressing Y-FAST and mCherry (HMBR channel: Ex/Em 491/525–539 nm; mCherry channel: Ex/Em 561/605–664 nm). HMBR concentration was 10 μM. See also Movie S3. (D) A zebrafish embryo expressing Y-FAST and mCherry was imaged before addition of HMBR (–HMBR), 20 min after incubation with 10 μM HMBR (+HMBR), after two washings of 20 min (Wash 1 and 2), and after reincubation with 10 μM HMBR (+HMBR). (E) Confocal micrographs of live HeLa cells expressing Dronpa−NLS (nucleus) and lyn11−Y-FAST (membrane) showing sequential imaging of nuclear Dronpa and membrane-anchored Y-FAST through sequential on/off labeling of Y-FAST intercalated with on/off photoswitching of Dronpa (Ex/Em 488/493–797 nm). HMBR concentration was 5 μM. (Scale bars, 10 μm.) |
|
Labeling of fusion proteins in zebrafish. (a) Spinning-disk confocal micrographs of live zebrafish embryos co-expressing mCherry/Y-FAST or mCherry/PYP labeled with 5 μM HMBR during gastrulation (HMBR channel: Ex/Em 491/525-539 nm, mCherry channel: Ex/Em 561/605-664 nm; scale bars 200 μm). Side-by-side images were recorded using the same settings. (b) Viability of zebrafish embryos incubated with HMBR during development from 50 % epiboly to 24 hpf (19 hours of incubation). The plot shows for various HMBR concentrations the number of embryos that were alive with no morphological defect (blue) or dead (red) at 24 hpf. |