- Title
-
Zebrafish as a Model to Investigate Dynamin 2-Related Diseases
- Authors
- Bragato, C., Gaudenzi, G., Blasevich, F., Pavesi, G., Maggi, L., Giunta, M., Cotelli, F., Mora, M.
- Source
- Full text @ Sci. Rep.
|
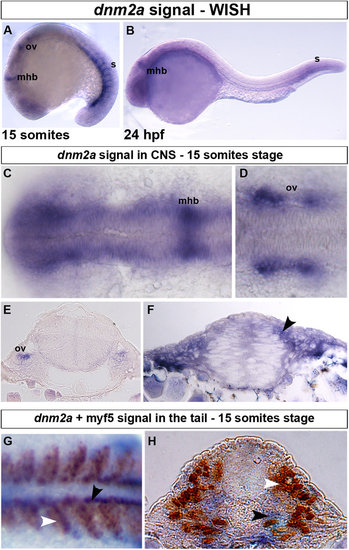
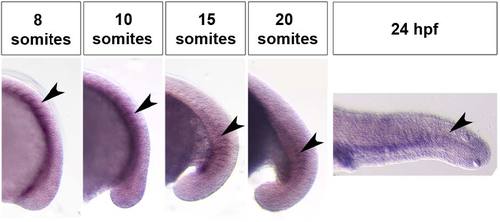
WISH and double-staining of dnm2a and myf5. WISH shows that dnm2a transcripts are present in the CNS and tail of zebrafish embryos from early somitogenesis (11 hpf approximately) to 30 somites stage (24 hpf). (A) At the 15-somite stage dnm2a is present in the tail (somites, s), at the midbrain/hindbrain boundary (mhb) and, bilaterally, at the two otic vesicles (ov). (B) At 24 hpf, dnm2a is present in newly formed somites and appears to be declining in intensity at the midbrain/hindbrain boundary. (C) WISH shows dnm2a signal in CNS, in embryos at 15 somites stage, more pronounced in the midbrain/hindbrain boundary and in the two bilateral otic vesicles (D,E). (F) The expression of dnm2a is diffuse in the neural tube, and more intense in the periventricular and in the dorso-lateral portion (black arrowhead). (G) Double-staining reveals dnm2a and myf5 at the 15 somite stage: dnm2a appears (in dorsal view of flat mounted embryo dnm2a in somites) posteriorly and close to the notochord (black arrowhead) overlapping to some extent with myf5 (white arrowhead); in cross section (H), dnm2a is present in the medio-ventral part of the somite and in several adaxial cells (black arrowhead), while myf5 is expressed in medial cells (white arrowhead). EXPRESSION / LABELING:
|
|
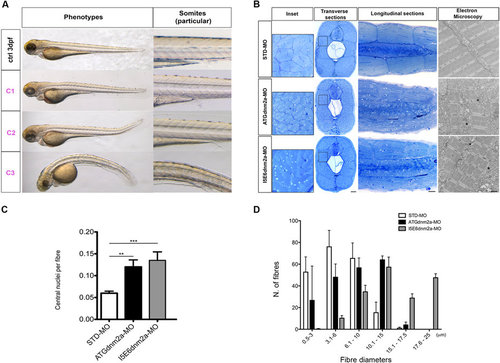
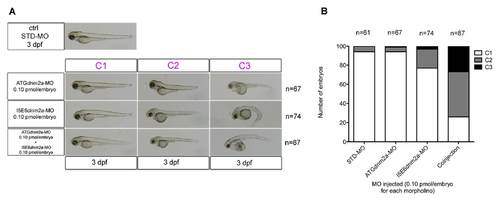
Morphological analysis and quantifications of morpholino-injected embryos. (A) Morphological features of STD-MO (ctrl) and morphants, observed under DMR microscope and subdivided into classes according to somite appearance: C1 completely formed, C2 partially disrupted, C3 unformed or totally disrupted somites (morphological features of ATGdnm2a-MO and I5E6dnm2a-MO-injected embryos are overlapping and representative images are shown). (B left) Toluidine blue-stained transverse and longitudinal sections at 4 dpf show evident muscle fibre disorganization in ATGdnm2a-MO and I5E6dnm2a-MO-injected embryos compared to STD-MO. Scale bar = 20 µm. (B right) Electron micrographs of longitudinal sections show myofibrils less regularly arranged, abundant membranous structures, vesicles and tubules (asterisks) in ATGdnm2a-MO and I5E6dnm2a-MO-injected embryos, compared to STD-MO. Scale bar = 1 µm. (C) Quantitation of central nuclei per fibre shows significantly more central nuclei in ATGdnm2a-MO and even more in I5E6dnm2a-MO-injected embryos than STD-MO. (D) Quantitation of fibre diameter indicates that the distribution of fibre diameters is shifted towards larger diameters in ATGdnm2a-MO and I5E6dnm2a-MO-injected embryos compared to STD-MO. Morphological analysis were performed on 6 embryos for each group (ATGdnm2a-MO, I5E6dnm2a-MO and STD-MO), chosen randomly from 6 independent injections. PHENOTYPE:
|
|
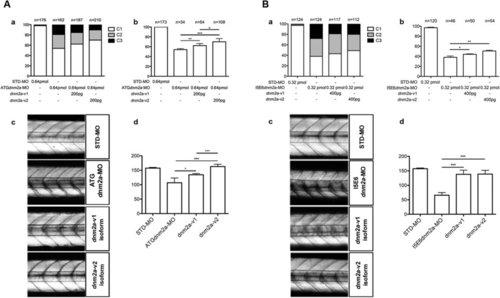
(A) Rescue with dnm2a-v1 and dnm2a-v2 after injection of ATGdnm2a-MO. (a) Appearance of embryos at 3 dpf after injection of ATGdnm2a-MO and either dnm2a-v1 or dnm2a-v2 rescue. The proportion of normal-appearing embryos (C1) is greater after rescue with dnm2a-v1 and dnm2a-v2 than after treatment with ATGdnm2a-MO alone. (b) Touch evoked response test results in normal-appearing embryos (C1) identified in experiments performed in (a). The percentage of embryos with normal touch evoked response was significantly greater in touch evoked after rescue with dnm2a-v1 and dnm2a-v2 than embryos injected with ATGdnm2a-MO alone (results obtained from 5 indipendent experiments). (c) Analysis of birefringence at 3 dpf in n = 3 somites after the end of the yolk in 6 independent replicates. Birefringence is evident in the muscle of STD-MO embryos, reduced in somites of ATGdnm2a-MO embryos and reverted to almost normal in embryos rescued with dnm2a-v1 and dnm2a-v2. (c) Graph shows quantitation of birefringence in STD-MO embryos, ATGdnm2a-MO embryos, embryos rescued with dnm2a-v1 and embryos rescued with dnm2a-v2. (B) Rescue with dnm2a-v1 and dnm2a-v2 after injection of I5E6dnm2a-MO. (a) Appearance of embryos at 3 dpf after injection of I5E6dnm2a-MO and either dnm2a-v1 or dnm2a-v2 rescue. The proportion of normal-appearing embryos (C1) is greater after rescue with dnm2a-v1 and dnm2a-v2 than after treatment with I5E6dnm2a-MO alone. (b) Touch evoked response test results in normal-appearing embryos (C1) identified in experiments performed in (a). The percentage of embryos with normal touch evoked response was significantly greater in touch evoked after rescue with dnm2a-v1 and dnm2a-v2 than embryos injected with I5E6dnm2a-MO alone (results obtained from 4 independent experiments). (c) Analysis of birefringence at 3 dpf in n = 3 somites after the end of the yolk in 6 independent replicates. Birefringence is evident in the muscle of STD-MO embryos, but significantly reduced in somites of I5E6dnm2a-MO embryos and reverted to almost normal in embryos rescued with dnm2a-v1 and dnm2a-v2. (c) Graph shows quantitation of birefringence of STD-MO embryos, I5E6dnm2a-MO embryos, embryos rescued with dnm2a-v1 and embryos rescued with dnm2a-v2. PHENOTYPE:
|
|
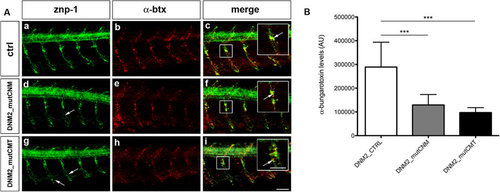
Primary motor neurons (znp-1) and AChRs (α-btx) in human mRNAs injected embryos. (A) Co-localization of markers for primary motor neurons (znp-1) and AChRs (α-btx) in 5 spinal hemisegments and somites, in 48 hpf zebrafish embryos. The images are representative of those found in n = 10 embryos for each condition, during 3 distinct experiments. In DNM2_mutCNM-injected embryos (d,e) and DNM2_mutCMT-injected embryos (g,h) primary motor axons migrate normally along the common path (compare a with d and g) with slight pathfinding and shape defects after the choice point (arrows). The merge images suggest that DNM2_mutCNM- and DNM2_mutCMT-injected embryos present fewer α-btx-positive spots than DNM2-controls, with the co-localization signal is reduced in intensity (f,i), compared to control (c), even though znp-1 and α-btx signals co-localize correctly (arrowhead in the enlargement, scale bar = 25 µm). Scale bar = 20 µm. (B) Quantification of α-btx-positive spots in n = 10 complete embryos for each condition. DNM2_mutCNM and DNM2_mutCMT- injected embryos present fewer α-btx-positive spots than controls. Error bars are SEMs. Scale bar = 20 µm. |
|
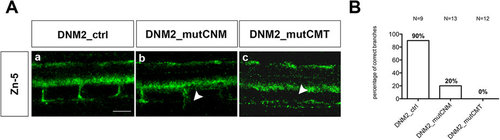
Secondary motor neurons (zn-5) in embryos injected with human mRNAs. (A)Visualization of secondary motor neurons by zn-5 monoclonal antibody-labelling of 48 hpf zebrafish embryos. By 48 hpf secondary motor neurons have completed their migration along the common path, and axons of the ventral nerve extend to the ventral myotome in both wild-type embryos (not shown) and those injected with wild-type DNM2 mRNA (DNM2_ctrl) (a). However in DNM2_mutCNM embryos, secondary motor neuron axons appear to exit the spinal cord and migrate to the periphery, but branching is rare (b). By contrast, in embryos expressing DNM2_mutCMT secondary motor axons are not observed (c). Scale bar = 10 µm. The images are representative of those found in average12 embryos for each condition, during 3 independent experiments. (B) Graph shows quantitation of branchings. |
|
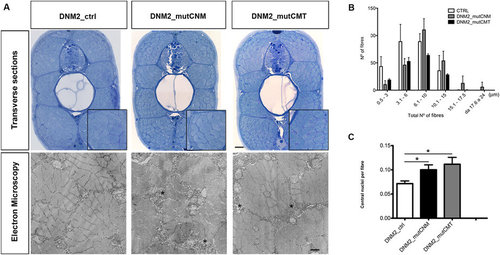
Morphological analysis and quantifications of human mRNAs injected embryos. (A) Toluidine blue-stained transverse sections and electron micrographs at 4 dpf of DNM2_mutCNM and DNM2_mutCMT -injected embryos in comparison to control (DNM2_ctrl). In Toluidine blue-stained sections both CNM and CMT injected embryos muscle tissue has more disordered morphology than control embryos, with fibres of variable size, frequent lobular appearance and more space surrounding fibres. Scale bar = 10 µm. Electron micrographs show greater vesicular decoration in spaces surrounding fibres (asterisks) and loose morphology. Scale bar = 0.5 µm. (B) Quantitation of fibre diameter indicates that the distribution of fibre diameter is shifted towards larger diameter in CNM- and CMT-injected embryos compared to control (in DNM2_mutCNM and DNM2_mutCMT 30.2% and 17.3% respectively of fibre diameters were 10.1 µm or greater, compared to 13.3%in control p < 0.0001). (C) Quantitation of central nuclei per fibre shows significantly more central nuclei in both CNM- and CMT-injected embryos than control. Morphological analysis were performed on n = 6 embryos for DNM2_mutCNM, DNM2_mutCMT and DNM2_ctrl each, chosen randomly from 4 different injections. |
|
WISH shows the dnm2a expression pattern during somitogenesis. In the tail, dnm2a expression varied within somite maturation. The dnm2a signal wad detected in newly formed somites, and progressively disappeared from more rostral ones. EXPRESSION / LABELING:
|
|
(A) Low doses (0.10 pmol/embryo) of ATGdnm2a-MO and I5E6dnm2a-MO were co-injected in the same embryos, compared with the injection of 0.10 pmol/embryo of STD-MO. (B) When combined, the morpholinos cause severe morphological alterations even at doses that were negligible on their own, confirming their targeting specificity. |

Unillustrated author statements PHENOTYPE:
|