- Title
-
Rotating pigment cells exhibit an intrinsic chirality
- Authors
- Yamanaka, H., Kondo, S.
- Source
- Full text @ Genes Cells
|
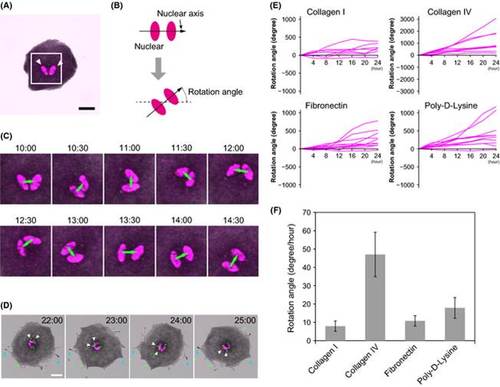
Zebrafish melanophores exhibit unidirectional counterclockwise rotation. (A) A melanophore harvested from the fins of Tg(mitfa:Histone H2B-RFP) fish. Histone H2B-RFP was localized in the nuclei (white arrowheads) Scale bar represents 20 µm. (B) Definition of the nuclear axis and the rotational angel. Nuclear axis is the axis that passed between the centroids of the nuclei (C) Magnified images of the rectangular region in (A) 10–14 h 30 min after medium exchange. Green arrows represent the nuclear axes. The nuclei rotated counterclockwise. See Movie S1 (Supporting Information). (D) The apical surface of the melanophores rotated with the nuclei. Green dots are fluorescent beads spread over the surface of the melanophores 1 h before medium exchange. White arrowheads indicate beads that rotated with the nuclei (magenta). Cyan arrowheads indicate the pseudopodia that maintained the same position throughout the observation period. Scale bar represents 20 µm. See Movie S2 (Supporting Information). (E) The rotation angles of melanophores on various matrix coating were observed for 24 h: collagen I (N = 8), collagen IV (N = 9), fibronectin (N = 11), and poly-D-Lysine (N = 10) (F) The mean rotational speed of the melanophores on various matrix coatings: collagen I (7.9°/h, SEM ± 2.8°, N = 8), collagen IV (47.0°/h, SEM ± 12.1°, N = 9), fibronectin (10.8°/h, SEM ± 2.8°, N = 11), and poly-D-Lysine (17.9°/h, SEM ± 5.6°, N = 10). All cells rotated in a counterclockwise direction, although the matrix material affected the rotational angle. |
|
Effect of cytoskeleton inhibitors on cellular rotation. (A) The angles of rotation of melanophores treated with cytoskeleton inhibitors were observed for 12 h: DMSO (dimethyl sulfoxide) treatment (N = 24), cytochalasin D treatment (N = 10), nocodazole treatment (N = 11), and nocodazole and cytochalasin D treatment (N = 20). See Movie S3–6 (Supporting Information). (B) Effect of cytoskeleton inhibitors on cellular rotation. Cytochalasin D effectively inhibited cellular rotation, whereas nocodazole accelerated cellular rotation. The mean rates of rotation of the melanophores were as follows: DMSO (dimethyl sulfoxide) treatment (21.4°/h, SEM ± 4.3°, N = 24), cytochalasin D treatment (2.9°/h, SEM ± 1.7°, N = 10), nocodazole treatment (39.1°/h, SEM ± 6.6°, N = 11), and nocodazole plus cytochalasin D treatment (3.4°/h, SEM ± 1.5°, N = 11). (C) The actin cytoskeleton and microtubule network of rotating melanophores. The control melanophores were round, with actin fibers localized in the peripheral region and microtubules radially extended from the central region (control). When microtubule formation was inhibited by nocodazole, the actin fibers accumulated in certain sites in the peripheral region. The melanophores became irregularly shaped (+nocodazole). Scale bars represent 20 µm. |
|
Actin dynamics has a pivotal role in chirality of cellular rotation. (A) A rotating melanophore from a Tg(mitfa: Histone H2B-RFP, mitfa:lifeact-GFP, golden) fish. The lower panels show magnified images of those in the upper panels. The numbers in the upper panels indicate the time after the medium was exchanged (h: min). Scale bars represent 20 µm. White arrowheads indicate a rotating nucleus (magenta). The actin fibers were visualized with Lifeact-GFP (green). White double-headed arrows indicate bundles of actin fibers, which rotated clockwise. See Movie S6 (Supporting Information). (B) A model of cellular rotation of melanophore. Red arrows represented the direction of cellular rotation. Note that the direction is counterclockwise from basement view. Green arrows represented the direction of growth of actin fibers. Blue arrows represented the direction of the movement of actin fibers. The newly synthesized actin fibers pushed apical cell body in anticlockwise direction. |