- Title
-
Orphan G-Protein Coupled Receptor 22 (Gpr22) Regulates Cilia Length and Structure in the Zebrafish Kupffer's Vesicle
- Authors
- Verleyen, D., Luyten, F.P., Tylzanowski, P.
- Source
- Full text @ PLoS One
|
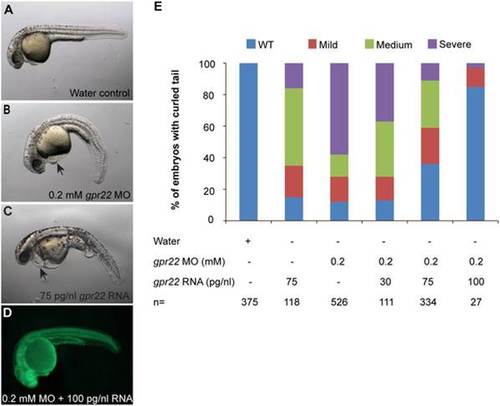
The downwards bending of the tail in gpr22 morphants is rescued by the co-injection of gpr22 mRNA. (A–C) Lateral views with anterior to the left of zebrafish embryos at 24 hpf injected at one cell stage with (A) water, (B) 0.2 mM fluorescein tagged gpr22 MO, (C) 75 pg/nl gpr22 mRNA or (D) 0.2 mM fluorescein tagged gpr22 MO and 100 pg/nl gpr22 mRNA. (B, C) Deregulation of gpr22 results in a bending of the tail, a reduction in length of the anterior-posterior axis and heart edema (arrow). (E) Quantification of the tail phenotype. (D, E) The curly tail of embryos injected with fluorescein tagged gpr22 MO is rescued by the co-injection of gpr22 mRNA in a dose-dependent manner. MO = morpholino, hpf = hours post-fertilization, n = number of analyzed embryos, WT = wild type. PHENOTYPE:
|
|
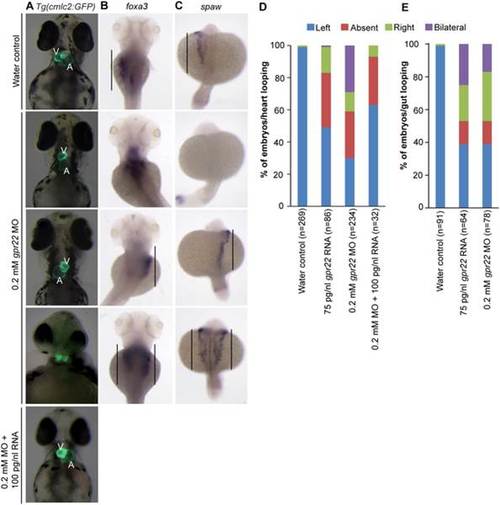
gpr22 deregulation results in defective LR patterning. (A) Ventral or (B, C) dorsal views with anterior to the top of embryos at (A, B) 4 dpf or (C) 20 somite stage. (A) Transgenic embryos expressing GFP under control of the cmlc2 promoter. In contrast to water controls, gpr22 morphants show (from top to bottom) normal, absent, reversed or bicardial cardiac looping. Co-injection of 0.2 mM gpr22 MO and 100 pg/nl gpr22 RNA partially rescues the randomized cardiac looping (last panel). (B) WISH for foxa3. The liver of water control embryos develops at the left side of the embryo. In contrast, the position of the liver in embryos injected with 0.2 mM gpr22 MO is randomized (straight line). (C) WISH for southpaw (spaw). gpr22 morphants show randomized expression of the early left-specific LPM marker southpaw (straight line). (D) Quantifications of the heart or (E) liver/gut phenotype. MO = morpholino, V = ventricle, A = atrium, dpf = days post-fertilization, n = number of analyzed embryos, GFP = green fluorescence protein, cmlc2 = cardiac myosin light chain type 2, LR = left-right, WISH = whole mount in situ hybridization, LPM = lateral plate mesoderm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
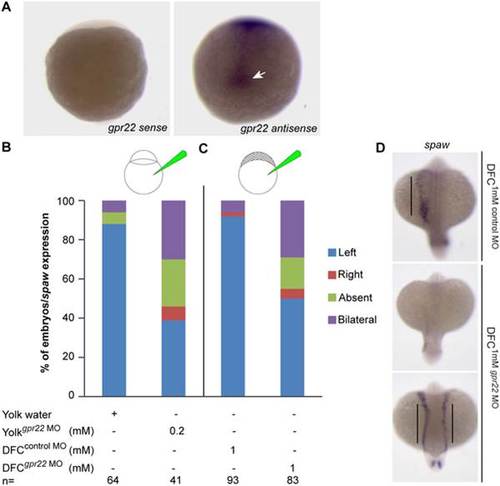
Gpr22 regulates LR asymmetry through its function in the KV. (A) WISH on 1–5 somite stage zebrafish embryos using the gpr22 sense or gpr22 antisense probe. Shown are the dorsal views of the tail bud region with anterior to the top. gpr22 is expressed in the axial structures and in the forming KV (arrow). (B, C) Percentage of embryos with randomized southpaw (spaw) expression, injected with (B) water or 0.2 mM gpr22 MO into the yolk at one cell stage (knock down in the whole embryo), or with (C) 1 mM control MO or 1 mM gpr22 MO into the yolk at mid-blastula stage (DFC/KV specific knock down). A DFC/KV specific knock down of gpr22 results in a similar percentage of zebrafish embryos showing randomized southpaw expression as a knock down in the whole embryo. (D) Representative images of panel (C). MO = morpholino, n = number of analyzed embryos, yolksuperscript = injected at one cell stage, DFCsuperscript = injected at mid-blastula stage, WISH = whole-mount in situ hybridization, DFC = dorsal forerunner cell, LR = left-right, KV = Kupffer’s vesicle. EXPRESSION / LABELING:
PHENOTYPE:
|
|
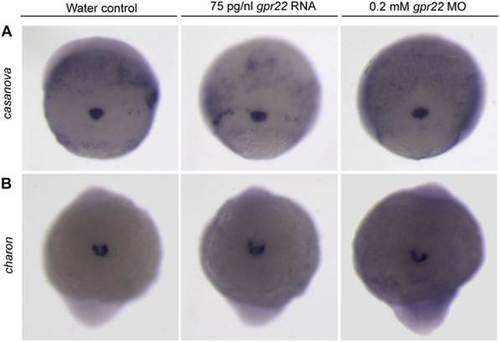
Gpr22 is not involved in KV formation or morphology. (A) WISH for the DFC marker casanova on embryos at 75% epiboly or (B) for the KV marker charon on 10 somite stage embryos. Shown is (A) the dorsal view (B) of the tailbud region with anterior to the bottom. gpr22 overexpression or knock down does not alter the expression of casanova or charon. MO = morpholino, KV = Kupffer’s vesicle, WISH = whole mount in situ hybridization, DFCs = dorsal forerunner cells. EXPRESSION / LABELING:
|
|
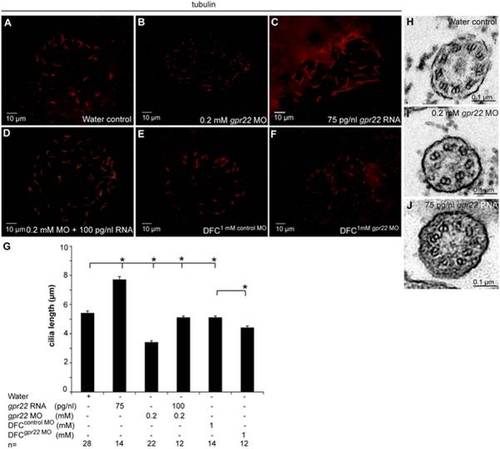
Gpr22 affects cilia length and structure in the KV. (A–F) Immunostaining with anti-acetylated tubulin antibody on embryos at 10 somite stage injected at (A–D) one cell stage with (A) water, (B) 0.2 mM gpr22 MO, (C) 75 pg/nl gpr22 RNA, (D) 0.2 mM gpr22 MO and 100 pg/nl gpr22 RNA, or injected at (E, F) mid-blastula stage with (E) 1 mM control MO, (F) 1 mM gpr22 MO. Shown are the cilia of the KV. (G) Quantification of average cilia length ± s.d. (B, G) Whole embryo or (F, G) DFC-specific knock down of gpr22 significantly reduces cilia length. (C, G) In contrast, gpr22 overexpression results in significantly longer cilia. (D, G) Co-injection of 0.2 mM MO and 100 pg/nl RNA almost completely rescues cilia length. (H–J) TEM of cross sections of the KV cilia at 10 somite stage. (H) Structure of water control KV cilia showing 9 parallel outer microtubule doublets. (I) gpr22 knock down or (J) overexpression results in a disruption of the proper microtubuli arrangement or (I) even an absence of doublets. MO = morpholino, n = number of analyzed embryos, DFC = dorsal forerunner cells, DFCsuperscript = injected at mid-blastula stage, KV = Kupffer’s vesicle, TEM = transmission electron microscopy, * = P<0.01 (unpaired, two-tailed T-test). PHENOTYPE:
|
|
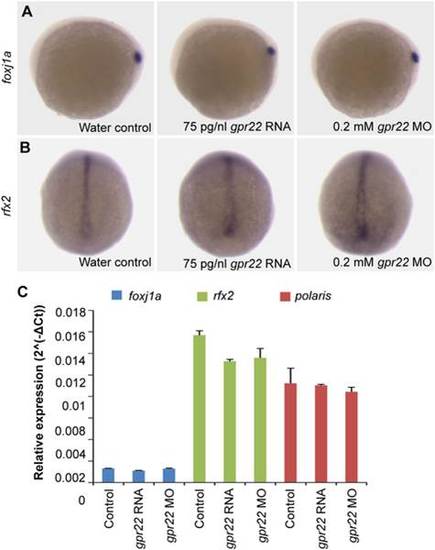
Gpr22 does not act upstream of Foxj1a/Rfx2 in KV ciliogenesis. (A, B) WISH of (A) foxj1a or (B) rfx2 on embryos at bud stage. Shown are (A) the lateral view with anterior to the left or (B) dorsal view with anterior to the top. gpr22 knock down or overexpression does not alter the DFC expression of foxj1a or rfx2, two master regulators of ciliogenesis. (C) Confirmation by qPCR of the foxj1a, rfx2 and polaris (target gene) RNA levels in the whole embryo, normalized to beta-actin. MO = morpholino, WISH = whole-mount in situ hybridization, DFC = dorsal forerunner cell, KV = Kupffer’s vesicle. EXPRESSION / LABELING:
|
|
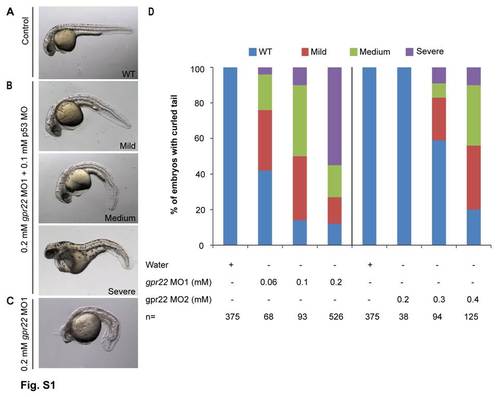
gpr22 knock down results in a dose-dependent curvature of the tail. (A–C) Lateral views with anterior to the left of zebrafish embryos at 24 hpf, injected at one cell stage with (A) water, (B) 0.2 mM gpr22 MO1 with 0.1 mM p53 MO or (C) without p53 MO. (D) Quantification of the tail phenotypes. (A, B, D) Knock down of gpr22 with either MO1 or MO2, results in a dose-dependent WT, mild, medium or severe downwards curvature of the tail. (B, C) Co-injection with p53 MO rescues the head necrosis caused by MO1. MO = morpholino, hpf = hours post-fertilization, n = number of analyzed embryos, WT = wild type. |
|
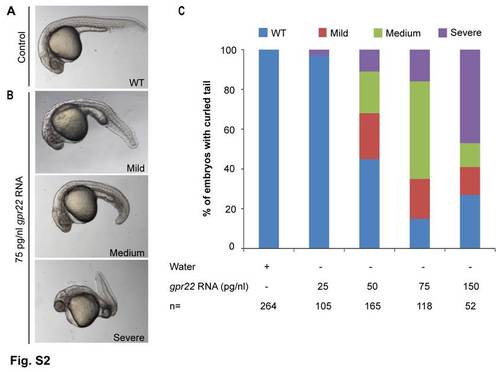
gpr22 overexpression results in a dose-dependent curvature of the tail. (A–C) Lateral views with anterior to the left of zebrafish embryos at 24 hpf, injected at one cell stage with (A) water or (B) 75 pg/nl gpr22 RNA. (C) Quantification of the tail phenotypes. (A–C) Overexpression of gpr22 results in a dose-dependent WT, mild, medium or severe curvature of the tail. hpf = hours post-fertilization, n = number of analyzed embryos, WT = wild type. |
|
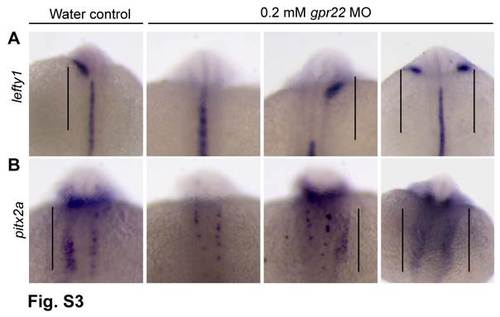
gpr22 knock down results in randomized expression of the left-specific LPM markers.(A, B) Dorsal views with anterior to the top of embryos at the 20 somite stage. (A) WISH for lefty1 or (B)pitx2a. The expression of the LR markers lefty1 and pitx2a is randomized in gpr22 morphants (straight line). LPM = lateral plate mesoderm, WISH = whole mount in situ hybridization.MO = morpholino. |
|
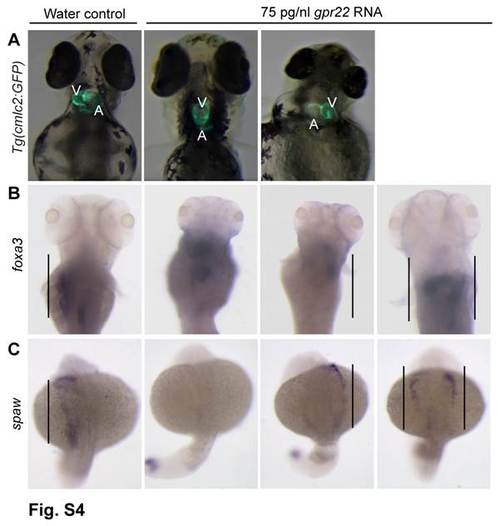
gpr22 overexpression results in defective LR patterning. (A) Ventral or (B, C) dorsal views with anterior to the top of embryos at (A, B) 4 dpf or (C) 20 somite stage. (A) Transgenic embryos expressing GFP under control of the cmlc2 promoter. gpr22 overexpression results in (from left to right) normal, absent, reversed or bicardial cardiac looping (not shown). (B) WISH for foxa3. The liver of water injected control embryos develops at the left side of the embryo. In contrast, the position of the liver in embryos injected with gpr22 RNA is randomized (straight line). (C) WISH for southpaw (spaw). The expression of the early LR marker southpaw is randomized in gpr22 injected embryos (straight line). V = ventricle, A = atrium, dpf = days post-fertilization, GFP = green fluorescence protein, cmlc2 = cardiac myosin light chain type 2, LR = left-right, WISH = whole mount in situ hybridization. |
|
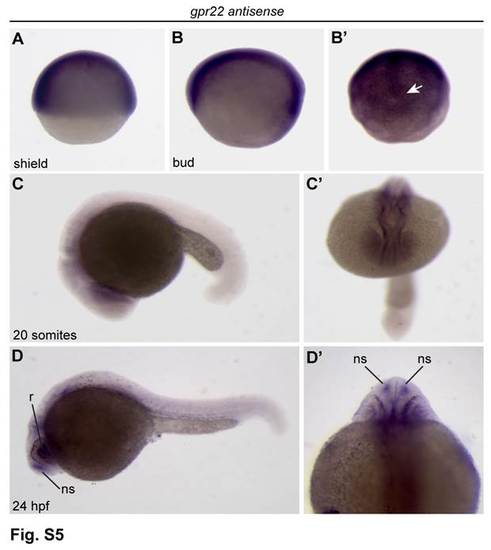
Expression analysis of gpr22 in zebrafish development.(A-D′) WISH for gpr22. (A, B, C, D) Lateral view with anterior to the left. (B′) Dorsal view of the tail bud region. (C′) Anterior view. (D′) Dorsal view with anterior to the top. (A, B) From shield to bud stage, gpr22 is ubiquitously expressed. (B′) From bud stage onwards, the expression pattern becomes more and more restricted to the axial structures and the developing KV (arrow). (C-D′) At later stages, gpr22 is expressed in several brain regions, the heart and some ciliated sensory organs, like (D) the retina and (D, D′) the nasal sac. WISH = whole-mount in situ hybridization, KV = Kupffer’s vesicle, hpf = hours post-fertilization, r = retina, ns = nasal sac. |
|
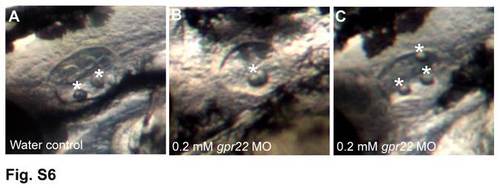
gpr22 morphants display otolith defects. Left ear with anterior to the left of zebrafish embryos at 2 dpf, injected at one cell stage with (A) water or (B, C) 0.2 mM gpr22 MO. (A) Control embryos have 2 tethered otoliths (white asterisks) at the anterior and posterior poles of the otic vesicle. In contrast, gpr22 morphants display (B) fused otoliths or (C) an increased number of otoliths, which are not correctly positioned. dpf = days post-fertilization, MO = morpholino. PHENOTYPE:
|