- Title
-
Divergence of zebrafish and mouse lymphatic cell fate specification pathways
- Authors
- van Impel, A., Zhao, Z., Hermkens, D.M., Roukens, M.G., Fischer, J.C., Peterson-Maduro, J., Duckers, H., Ober, E.A., Ingham, P.W., and Schulte-Merker, S.
- Source
- Full text @ Development
|
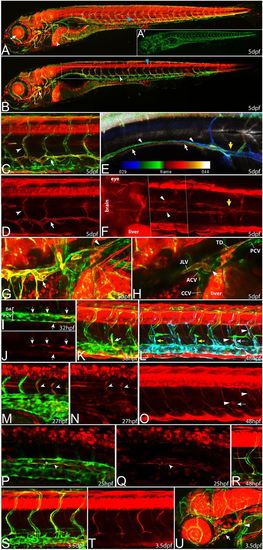
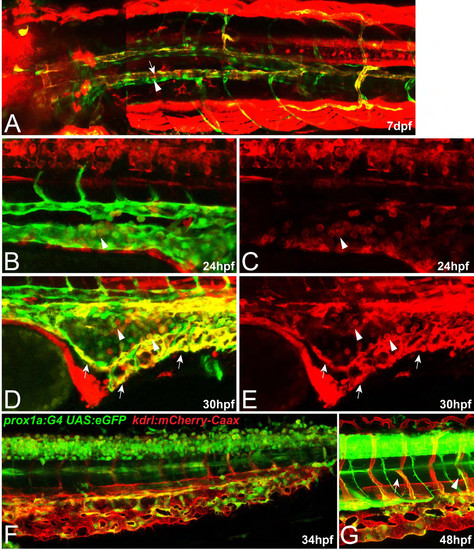
prox1a expression marks different endothelial compartments during vascular development. In all pictures (except for E), flt4:mCit expression is shown in green and prox1a:KalTA4,UAS:tagRFP expression is highlighted in red. (A) In full z-projections, prox1a expression at 5 dpf is apparent in various tissues, including liver (white arrowhead), lens (arrow) and myotome (blue arrowhead). (A2) Same z-projection displaying only the flt4:mCit expression in venous and lymphatic ECs. (B) Partial z-projection of the same embryo (comprising only optical sections without myotome signal) reveals additional expression in the spinal cord (blue arrowhead) and in the lymphatic vasculature of the head (arrow) and the trunk (white arrowhead). Images in A and B are composed of several overlapping z-projections because the embryo was too large to fit in a single field of view. (C,D) Higher magnification of trunk lymphatics at 5 dpf exhibiting combinatorial prox1a (red) and flt4 (green) reporter expression restricted to the TD (arrows) and ISLVs (arrowheads). (E) Depth color-coded z-projection (projecting each slice in a different color according to its position within the stack; see color bar) of boxed region in B (only prox1a channel) reveals the presence of two separate prox1a-positive vessels above the swim bladder (white arrows point to lymphatics on the right, arrowheads to lymphatics on the left body side), which merge near the sixth ISVs with the TD in the trunk region (yellow arrow). (F) Dorsal view of the prox1a-positive bilateral anterior TD (arrowheads), which connects to the trunk TD at the indicated location (yellow arrow). The image has been assembled from a set of partial z-projections (see dotted lines) owing to interference of other prox1a-positive structures. (G,H) Full (G) and partial (H) z-projections of the area (compare with box in A) where the anterior TD (arrowhead in G) and the facial lymphatic network (arrow in G) fuse and drain into the common cardinal vein (arrow in H) in a 5 dpf embryo. ACV, anterior cardinal vein; CCV, common cardinal vein; JLV, jugular lymphatic vessel; PCV, posterior cardinal vein; TD, thoracic duct. (I,J) At 32 hpf, prox1a-positive cells (red, arrows) are located within the PCV (green). Note that in this lateral view, prox1a-positive cells are located in both the dorsal and ventral aspect of the PCV. (K) A sprouting venous EC (arrow) at 38 hpf expressing both flt4 and prox1a transgenes. (L,O) At 48 hpf, only a proportion of PLs are prox1a:KalTA4-positive (white arrows) whereas the majority shows no signs of reporter expression (yellow arrows). In addition, venous ISVs positive for the prox1a reporter are evident (white arrowheads). (L) Overlay of a full z-projection of the flt4:mCit signal (green, to outline the complete vasculature) and identical partial z-projections of the prox1a (red, see also O) and flt4 reporter (blue, to highlight the part of the vasculature that is included in the partial z-projection of the prox1a signal). Note that prox1a-positive ECs will appear white in the overlay. (M,N) The prox1a reporter line also labels individual arterial sprouts (arrowheads) at 27 hpf. (P,Q) Single z-plane of the trunk region in a 25 hpf embryo showing DA cells positive for prox1a reporter expression (arrowheads). Note ventral domain expression of the DA. (R) Intersegmental vein showing both prox1a and flt4 reporter expression at 48 hpf. (S,T) At 3.5 dpf, all LECs display expression of the prox1a reporter but expression in other endothelial domains has disappeared. (U) Expression of prox1a in the head region at 3.5 dpf, highlighting the facial lymphatic network (arrow) including the drainage point with the common cardinal vein (arrowhead). EXPRESSION / LABELING:
|
|
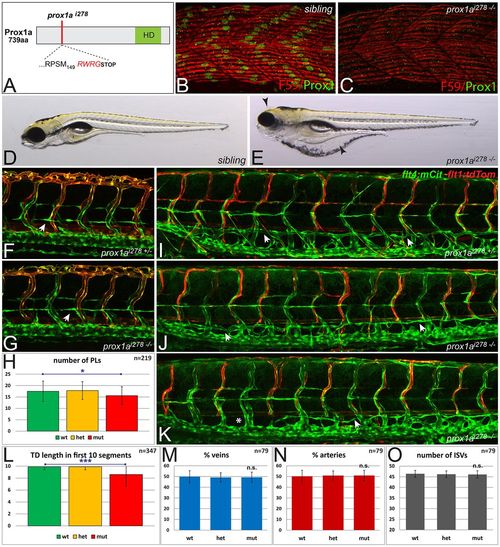
Lymphangiogenesis in prox1a mutant embryos. (A) Schematic of the homeodomain (HD) containing Prox1a protein, indicating the predicted effect of the 10 bp deletion in the prox1ai278 allele, leading to a frame-shift (red amino acids) and a truncated protein after 153 amino acids. (B,C) Prox1a immunostaining of slow muscle fibers in sibling (B) and homozygous mutant prox1ai278 (C) embryos demonstrates a complete loss of wild-type Prox1a protein (green) at 30 hpf (slow myosin heavy chain-1 is shown in red). (D,E) Brightfield pictures of 5 dpf sibling (D) and homozygous prox1ai278 mutant (E) embryos. Note the strong edema formation around the eye and gut area (arrowheads), which can be even more pronounced in other prox1a mutants at this stage. (F,G) In both heterozygous siblings (F) and homozygous prox1ai278 mutants (G), PLs appear at the level of the horizontal myoseptum at 2 dpf (arrows). (H) Average PL numbers per embryo are mildly reduced in prox1a mutants at 2 dpf (Student’s t-test, *P=0.025). Error bars indicate s.d. of wild-type (green), heterozygous (orange) and mutant (red) groups in embryos from a prox1a+/- incross. (I-K) flt4:mCit; flt1enh:tdTom double transgenic embryos highlighting arterial ISVs in red and venous and lymphatic structures in green. Compared with heterozygous siblings (I), most homozygous prox1ai278 mutants do not display TD defects at 5 dpf (J), whereas others display a mild reduction (K) in some areas of the trunk (arrows point at TD; asterisks mark the lack of TD). Note the overall unaffected ratio of venous and arterial ISVs in mutants (J,K). (L) Average number of segments positive for TD cells, scored in the first ten segments above the yolk extension at 5 dpf. Error bars indicate the s.d. for the respective genotypic class from a prox1a+/- incross. ***P=2.3E-08 (Student’s t-test, comparison of wild-type and mutant population). (M,N) The average percentage of intersegmental veins (M) and arteries (N) does not differ between genotypic classes in an incross of prox1ai278 carriers. (O) In prox1ai278 mutants, the average number of ISVs is not altered. Error bars represent s.d. n.s., not statistically significant. |
|
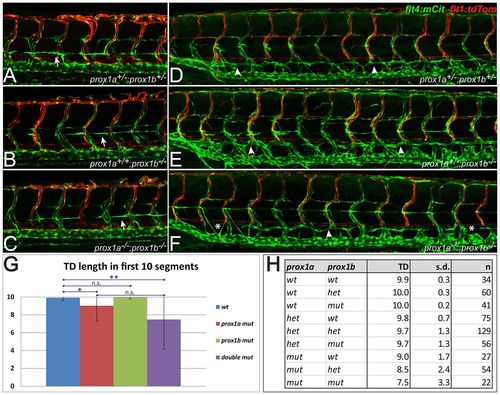
Lymphatic specification is not blocked in prox1ai278;prox1bSA0035 double mutants. (A-C) In all genotype combinations of a prox1ai278;prox1bSA0035 double heterozygous incross, including double heterozygotes (A), homozygous prox1b mutants (B) and homozygous double mutants (C), PLs are apparent at the horizontal myoseptum at 2 dpf (arrows). (D-F) As in double heterozygous embryos (D), the loss of both copies of prox1b (E) does not lead to lymphatic defects on the level of TD formation. In prox1ai278;prox1bSA0035 double homozygous embryos (F), mild TD defects are occasionally visible (arrows indicate the TD and asterisks highlight segments without TD). (G) Average number of TD-positive segments within the first ten segments above the yolk extension for the indicated genotypic classes of a prox1a+/-;prox1b+/- incross. The moderate reduction in TD length is statistically significant in the prox1a single mutants (Student’s t-test, *P=0.01) and the prox1a;prox1b double mutant embryos (**P=0.002) when compared with wild-type siblings. Error bars represent s.d. (H) Overview about the average TD length (TD) in ten scored segments, the corresponding s.d. and the number of scored embryos (n) in a prox1a+/-;prox1b+/- incross. n.s., not statistically significant. |
|
Specification of the lymphatic lineage is independent of coup-TFII and sox18. (A) Schematic of the Coup-TFII protein indicating the position of the first affected amino acid in the 1 bp insertion allele nr2f2hu10330 (coup-TFIIhu10330 hereafter) which leads to a premature stop codon after an additional four amino acids N-terminal to the nuclear receptor-DNA binding and ligand-binding domain. (B,C) Brightfield images of wild-type (B) and homozygous mutant coup-TFIIhu10330 (C) embryos, with no signs of edema or morphological abnormalities at 6 dpf. (D,E) flt4:mCit;flt1enh:tdTom-positive embryos from a coup-TFIIhu10330 incross. Compared with heterozygous siblings (D), coup-TFII mutants (E) show only marginal TD defects (asterisk) in a small proportion of embryos at 5 dpf. Note the overall normal vascular morphology with proper PCV, DA and ISVs present in homozygous mutants. Arrowheads indicate the presence of TD. (F) The average length of the TD in the first ten segments above the yolk extension is not significantly affected in coup-TFIIhu10330 mutants. (G) The proportion of venous ISVs is unaltered in homozygous coup-TFIIhu10330 mutants at 5 dpf. (H) Schematic overview of the Sox18 protein, indicating the first affected amino acid in the 1-bp insertion allele sox18hu10320, preceding the HMG-box. The resulting frame-shift leads to a premature stop codon after additional 50 amino acids. (I,J) Bright-field images of heterozygous (I) and homozygous (J) mutant sox18hu10320 embryos at 5 dpf. Note the overall wild-type appearance of mutant embryos (J). (K) The average length of the TD in wild-type (green), heterozygous (yellow) and homozygous mutant (red) sox18hu10320 embryos does not differ significantly. (L,M) sox18hu10320 heterozygous (L) and homozygous (M) mutant embryos expressing fli1a:eGFP in all ECs. In a sox18 loss-of-function situation, embryos do not show defects in the formation of the TD (arrowheads). (N) The average number of PLs present in sox18hu10320 mutants is not significantly affected at 54 hpf. Error bars represent s.d. n.s., not statistically significant. |
|
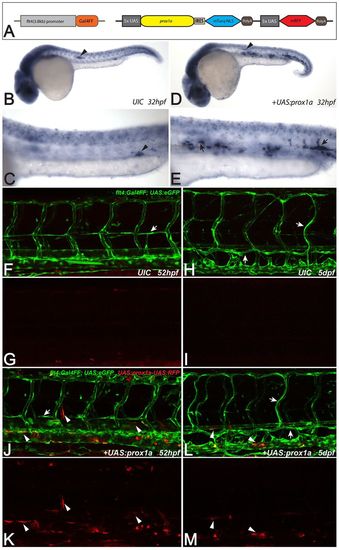
Forced expression of prox1a does not commit venous ECs to a lymphatic phenotype. (A) Schematic of the prox1a overexpression construct and the flt4:Gal4FF line, which drives Gal4FF expression under the control of a Medaka 3.8 kb flt4 promoter fragment. (B-E) Whole-mount in situ hybridization against prox1a in uninjected flt4:Gal4FF siblings (B,C) and embryos injected with the prox1a mis-expression construct at 32 hpf (D,E). Note the domain of forced prox1a expression within the axial vessels in injected embryos (arrows in E), which does not reflect endogenous prox1a expression (C). Arrowheads in B,D highlight endogenous prox1a expression in the lateral line primordium; arrowhead in C points at a signal in the corpuscles of Stannius. (F-M) flt4:Gal4FF;UAS:eGFP wild-type siblings (F-I) and embryos injected with UAS:prox1a_IRES_mTurq-NLS-UAS:mRFP plasmid (J-M). At 2 dpf, forced expression of prox1a in ECs (marked by mRFP expression in J,K) does not lead to the emergence of ectopic secondary sprouts (F,G). At 5 dpf, UAS:prox1a-UAS:mRFP positive ECs are still evident in arterial and venous ECs without any signs of lymphatic or venous defects (L), suggesting that prox1a expression is not capable of influencing the cell fate and behavior of arterial and venous ECs (red in L,M). Arrows in F,J point at PLs. In H,L, arrows highlight the TD and ISLVs and white arrowheads in J-M mark exemplary ECs harboring the prox1a mis-expression construct. Note that the flt4:Gal4FF reporter line shows occasional expression in myotome cells as well as in a subset of neurons in the spinal cord. UIC, uninjected control. |
|
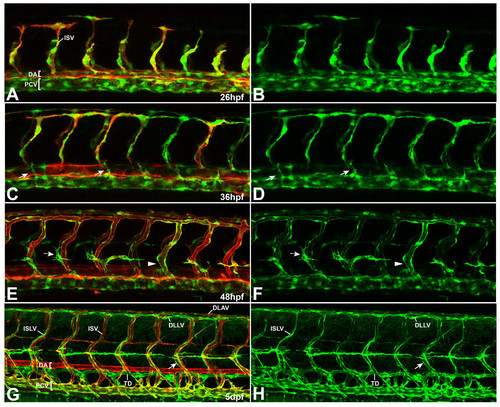
Expression pattern of flt4BAC:mCitrine reporter line at different stages of vascular development. (A-H) Double transgenic embryos for flt4:mCit (green) and kdrl:mCherry-Caax (red) at different stages of vascular development. (A, B) Initially, the flt4 reporter shows expression in both, arterial and venous ECs with an enriched signal within the venous compartment (26 hpf). (C, D) From about 26 hpf onwards, arterial expression of the construct decreases, so that emerging secondary sprouts can be easily followed at around 36 hpf (arrows). (E, F) At 2 dpf, the flt4 reporter expression is strongly confined to venous derived structures (venous ISV marked by arrowhead) and the signal gradually increases in the lymphatic lineage (see PLs highlighted by arrows). (G, H) By day 5, flt4:mCit expression is still evident in the PCV and venous ISVs (arrow). In addition, lymphatic structures including the TD, ISLVs as well as the DLAV are clearly highlighted throughout the trunk. [DA-dorsal aorta, PCV-posterior cardinal vein, TD-thoracic duct, ISV-intersegmantal vessel, ISLV-intersegmental lymphatic vessel, DLAV-dorsal longitudinal anastomotic vessel, DLLV-dorsal longitudinal lymphatic vessel] EXPRESSION / LABELING:
|
|
Additional expression domains of the prox1a reporter line (A-E) Prox1a reporter expression is shown in red, flt4:mCit expression is marked in green. (F, G) kdr-l expression domains are highlighted in red while expression of the prox1a reporter line is shown in green. (A) Dorsal view on the anterior part of the TD which splits up in two vessels (arrow) closely aligning to the likewise bilateral PCV (arrowhead) in a 7 dpf embryo. The image has been assembled from two overlapping partial z-projections (see dotted line). (B-E) At 24 hpf, before the onset of circulation, as yet undefined round cells within the axial vessels display expression of both markers (arrowheads in B, C). At 30 hpf, similar cells can still be seen with in the cardinal vein region (arrowhead in D, E). In addition, strong expression of the prox1a reporter is evident in a subpopulation of caudal vein cells (arrows in D, E). (F, G) prox1a is expressed within a subgroup of caudal vein cells at 34 hpf which most likely results in the higher amounts of prox1a positive venous (arrowhead) and lymphatic secondary sprouts (arrow) in this area at 48 hpf (G). Image F is composed of two overlapping z-projections. |
|
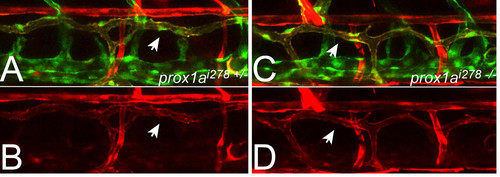
Expression of the prox1a reporter line in prox1ai278 mutants. (A-D) prox1a:KalTA4,UAS:tagRFP (TD in red), flt1enh:tdTom (DA and arterial ISVs in red) and flt4:mCit positive triple transgenic embryos at 5 dpf. As in heterozygous siblings (A, B) prox1a reporter expression also marks the TD (see arrows) in homozygous prox1ai278 mutants (C, D), suggesting that Prox1a is not required for the maintenance of its own expression. |
|
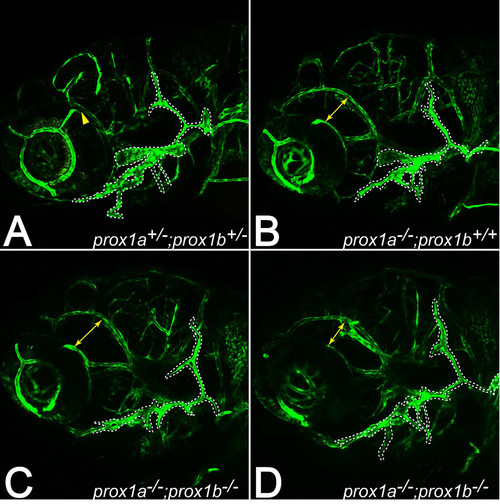
Facial lymphatics are formed in prox1ai278 and prox1ai278;prox1bSA0035 double mutants. (A-D) Facial lymphatic network in flt4:mCit expressing embryos at 5dpf. As in wild-type siblings (A), the facial lymphatics (outlined by dotted lines) also emerge in prox1a single (B) and prox1a;prox1b double mutant embryos (C-D), but seem to be less developed compared to wild-type structures at the same age. Yellow arrows indicate the distance between the dorsal ciliary vein and the primordial midbrain channel that is enlarged in prox1a-/- embryos due to massive eye edema (also see Fig. 2E). |