- Title
-
The Leukemia-Associated Mllt10/Af10-Dot1l Are Tcf4/{beta}-Catenin Coactivators Essential for Intestinal Homeostasis
- Authors
- Mahmoudi, T., Boj, S.F., Hatzis, P., Li, V.S., Taouatas, N., Vries, R.G., Teunissen, H., Begthel, H., Korving, J., Mohammed, S., Heck, A.J., and Clevers, H.
- Source
- Full text @ PLoS Biol.
|
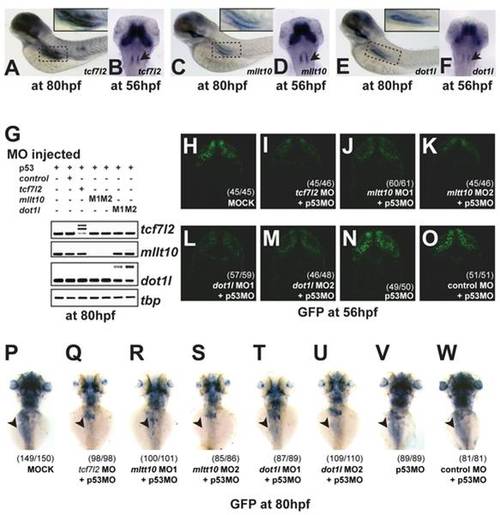
tcf7l2, Mllt10/Af10, and dot1l are co-expressed in Wnt active tissues and required for TCF-driven transcription in vivo. EXPRESSION / LABELING:
PHENOTYPE:
|
|
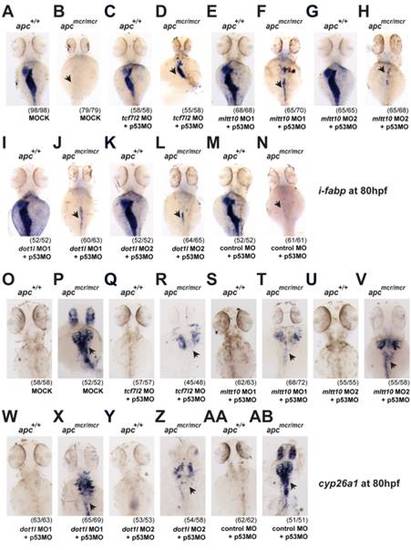
Depletion of mllt10/af10 and dot1l rescues intestinal defects in apcmcr/mcr zebrafish, mimicking tcf7l2 depletion. EXPRESSION / LABELING:
PHENOTYPE:
|
|
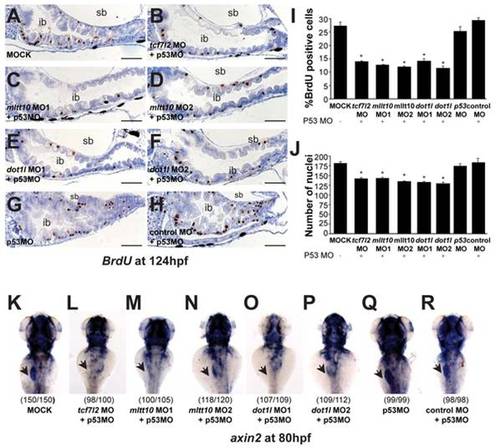
tcf7l2, mllt10/af10, and dot1l are essential for Wnt target gene expression and intestinal homeostasis in zebrafish. EXPRESSION / LABELING:
PHENOTYPE:
|
|
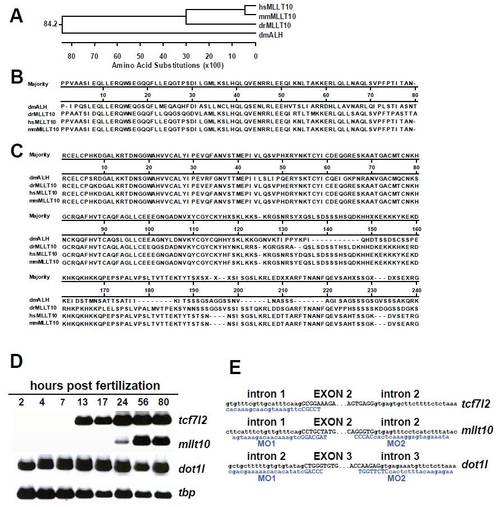
mRNA expression of tcf7l2, mllt10, and dot1l during zebrafish embryonic development and their depletion by splice-inhibiting MO sequences. (A) Phylogenetic tree analysis places zebrafish (Danio rerio) Mllt10 close to mouse and human sequences. (B–C) Amino acid sequence alignment of the Leucine zipper and PHD finger domains show high conservation of Mllt10 between species. (D) RT-PCR analysis of tcf7l2, mllt10, dot1l, and tbp RNA expression levels in whole embryos at different stages of embryonic development. (E) Representation of the splice-blocking MO sequences (blue) generated to deplete mRNA levels for tcf7l2, mllt10, and dot1l. |
|
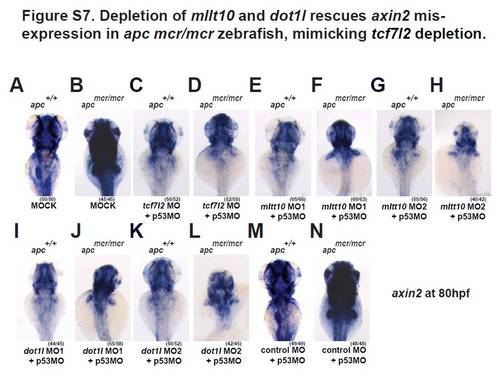
Depletion of tcf7l2, mllt10/af10 and dot1l rescues mis-expression of axin2 in apcmcr/mcr zebrafish, placing these genes downstream of Apc as Wnt target gene activators (A–N). Representative whole mount in situ hybridizations for axin2 in wild type and apcmcr/mcr mutant embryos at 80 hpf injected with (A,B) buffer alone, (C,D) MO against tcf7l2, (E–H) two independent mllt10/af10 MOs, (I–L) two independent dot1l MO, and (M,N) control MO. All MOs have been coinjected with a MO against p53. All images were captured using the same exposure and represent at least three independent experiments. In parentheses number of embryos showing described phenotype per number of total embryos analyzed. |
|
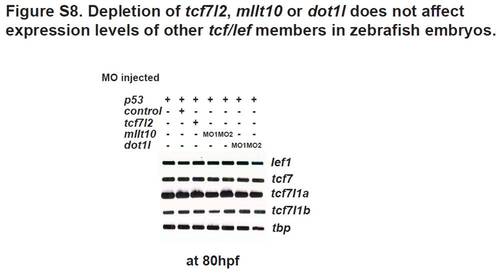
Depletion of tcf7l2, mllt10, or dot1l does not affect the expression levels of other tcf/lef family members in zebrafish embryos. RT-PCR analysis of lef1, tcf7, tcf7l1a, tcf7l1b, and tbp RNA expression levels in whole embryos injected with MO against p53 alone, or MO against p53 coinjected with MOs against tcf7l2, mllt10, dot1l, or control MO, respectively. |