- Title
-
The Zebrafish Embryo: A Powerful Model System for Investigating Matrix Remodeling
- Authors
- Wyatt, R.A., Keow, J.Y., Harris, N.D., Haché, C.A., Li, D.H., and Crawford, B.D.
- Source
- Full text @ Zebrafish
|
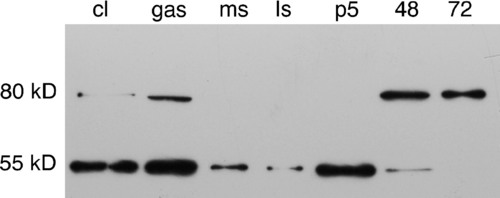
Western blot of staged whole embryo homogenates probed with anti-zebrafish MMP11. Whole embryo homogenates made from cleavage (cl), gastrulation (gas), mid-somitogenesis (ms), late somitogenesis (ls), primordium-5 (p5), 48-h, and 72-h embryos were western blotted and probed with anti-zebrafish MMP11. A 55kD band corresponding to the predicted molecular weight of MMP11 is detected in all stages up to 48 hpf, but both early and late stages of development express an antigenic band about 25kD larger than expected. This high-molecular-weight form of the antigen may represent complex formation or posttranslational modification of the antigen. MMP, matrix metalloproteinase. |
|
Immunostaining of MMP11 protein in a 48-hpf zebrafish embryo. Projections of confocal stacks were assembled to show the complete staining pattern of anti-zebrafish MMP11 in a 48-hpf embryo. Strong labeling is detected at maturing somite boundaries. Individual migrating mesenchymal cells are also labeled throughout the head and trunk (anterior to the left). |
|
Differential in vivo zymography with two peptides displaying differential susceptibility to MMP-mediated hydrolysis. This embryo was injected with a mixture of two MMP substrates (substrate I and substrate VI), whose breakdown products fluoresce in either the red or green channel, respectively. Both substrates are susceptible to hydrolysis by MMPs 2, 9, 13, and 14, but with different relative and absolute efficiencies. In the red channel, the fluorescent breakdown products of MMP-substrate peptide I are observed primarily in the brain, the vitelline circulation, squamous epithelial cells scattered across the surface of the embryo, the hatching gland, cells in the dorsal neural tube, maturing myotome boundaries, and the perichordal sheath. In the green channel, the fluorescent breakdown products of MMP-substrate peptide VI are observed primarily in the hatching gland, brain, myotome boundaries, cells of the dorsal neural tube, and the perichordal sheath. This suggests that sites where both peptides are similarly degraded (brain, myotome boundaries, cells of the dorsal neural tube, and notochord sheath) may harbor active MMPs 2 and/or 9, to which both peptides are similarly susceptible. Sites where one substrate is degraded significantly faster than the other must harbor protease(s) that preferentially degrade one of these substrates. In the scattered squamous epithelial cells and the cells in the vitelline circulation where substrate I is preferentially degraded, it is likely that MMP14, to which substrate I is more susceptible, is active. In contrast, in the hatching gland, where substrate VI is preferentially degraded, it is likely that MMP13, to which it is more susceptible, is the dominant protease. (Color figure is available at liebertonline.com.) |
|
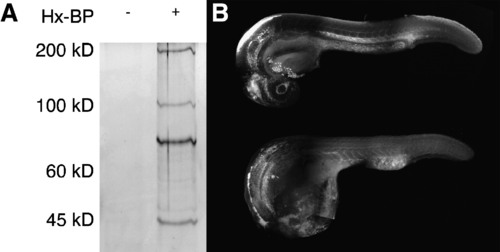
Activity based protease labeling in zebrafish embryos. (A) Embryonic homogenates were mixed with the hydroxamate-based MMP probe (Hx-BP) carrying a biotin moiety, crosslinked by ultraviolet irradiation, and the biotinylated proteins collected by precipitation with streptavidin beads. The precipitated proteins were then resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and detected by silver staining. The left (blank) lane shows that no proteins from homogenates are bound nonspecifically by the beads, but in the right lane, strong bands at roughly 50, 72, 95, and 190kD are apparent, suggesting that Hx-BP is able to biotinylate proteins of these molecular weights. (B) Embryos were injected with Hx-BP carrying a rhodamine moiety, and then either crosslinked by ultraviolet irradiation (top), or left unirradiated (bottom) before fixation and examination by confocal microscopy. The crosslinked embryo shows strong rhodamine fluorescence in the ventricles of the brain, hatching gland, the mesenchymal regions of the head, surrounding the lens, myotome boundaries, the perichordal sheath, and the extracellular matrix separating the mesoderm from the ectoderm in the elongating tail. The unirradiated embryo shows strong fluorescence in the ventricles of the brain, and weak ubiquitous fluorescence elsewhere. This suggests that the Hx-BP probe is being specifically localized at sites where active MMPs are remodeling the extracellular matrix in the embryo. |