- Title
-
Repression of RNA polymerase II elongation in vivo is critically dependent on the C-terminus of Spt5
- Authors
- Chen, H., Contreras, X., Yamaguchi, Y., Handa, H., Peterlin, B.M., and Guo, S.
- Source
- Full text @ PLoS One
|
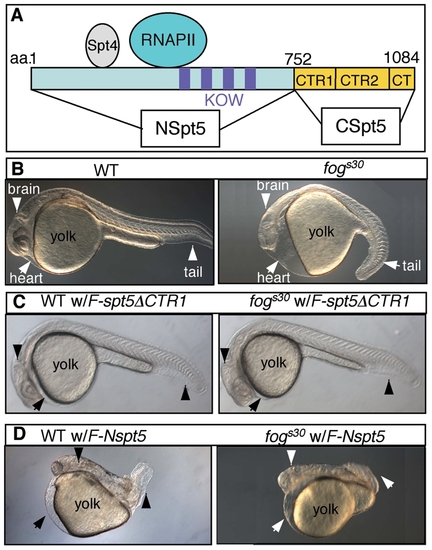
Injection of F-Nspt5 RNA into WT dominantly impairs embryonic development in zebrafish. (A) The functional domains of Spt5 based on previous in vitro analysis [15], [37]. (B–E) Morphological phenotypes of WT or fogs30 embryos (B), WT or fogs30 embryos injected with F-spt5 RNA (C), F-spt5ΔCTR1 RNA (D), or F-Nspt5 RNA (E). PHENOTYPE:
|
|
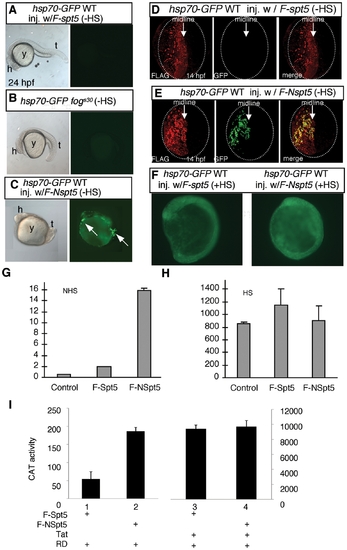
NSpt5 de-represses hsp70-4 expression in the absence of heat shock. (A–C) Embryonic morphology or GFP fluorescence of hsp70-GFP transgenic embryos. hsp70-GFP transgenic WT injected with F-spt5 RNA (A), hsp70-GFP transgenic fogs30 mutant (B), and hsp70-GFP transgenic WT injected with F-Nspt5 RNA (C). (D–E) Confocal images of FLAG- and GFP- double immuno-labeled embryos, injected with F-spt5 RNA (D), or with F-Nspt5 RNA (E). (F) GFP fluorescence in hsp70-GFP transgenic embryos injected with F-spt5 RNA (left) or F-Nspt5 RNA (right) and subjected to heat shock for one hour. (G–H) Quantitative RT-PCR analysis shows de-repression of hsp70-4 expression in 6 hpf Nspt5-expressing embryos (G), and no significant difference of hsp70-4 expression between F-Nspt5-expressing, F-Spt5-expressing, and control embryos upon heat shock (H). (I) F-NSpt5 increases transcription from the HIVLTR. CAT activity of Hela cells that express RD and F-Spt5 (lanes 1 and 3), or F-NSpt5 (lanes 2 and 4), in the absence (lanes 1 and 2) or presence of Tat (lanes 3 and 4). Results are presented in arbitrary units. Error bars represent S.E.M. from three independent experiments. EXPRESSION / LABELING:
|
|
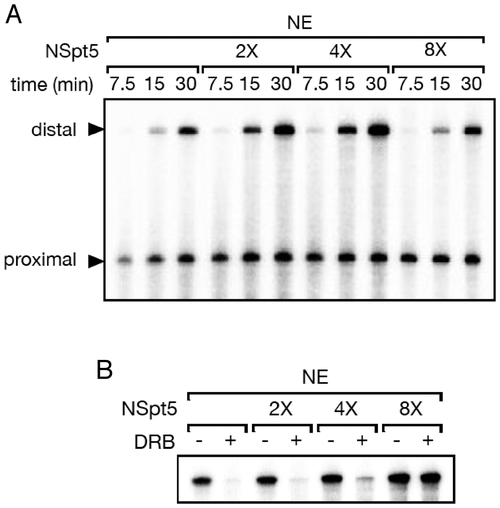
NSpt5 interferes with the repressive but not the stimulatory activity of endogenous DSIF in a dominant manner in vitro. (A) The elongation stimulation activity of DSIF was assayed using pSLG402 as a template, which generates short (promoter-proximal) and long (promoter-distal, dependent on the elongation stimulatory activity of DSIF) RNase T1-resistant products under the control of the adenovirus major-late promoter. Transcription initiation/elongation was allowed to proceed for the indicated times. Time-dependent increase of distal transcripts is observed in the control (first three lanes), while NSpt5 slightly enhanced transcription at 2X or 4X concentration but not at 8X concentration. (B) pTF3-6C2AT, which generates a 380-nt RNase T1-resistant product under the control of the adenovirus E4 promoter, was used as a template, and transcription was allowed to proceed for 10 minutes. This product is sensitive to the elongation repressive activity of DSIF (in the presence of the P-TEFb inhibitor DRB)(first two lanes). NSpt5 inhibits the repression activity of endogenous DSIF at 8X concentration. |
|
Morphological phenotypes of WT or fogs30 embryos injected with F-spt5 RNA. Injection of F-spt5 RNA fully rescues fogs30 embryo (right), but does not interfere with embryonic development in zebrafish WT embryo (left). |
|
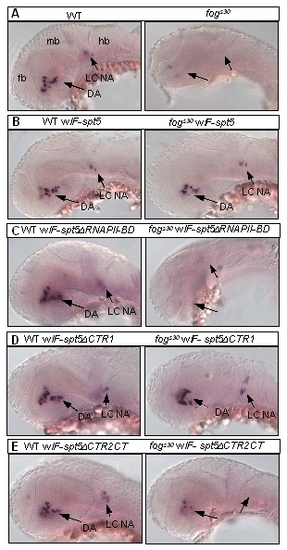
The effect of Spt5 deletion variants on DA neuron development. All images are lateral view of embryos in situ hybridized with the tyrosine hydroxylase (th) probe. Anterior is to the left, and dorsal is up. Abbreviations: DA, dopaminergic neurons; fb, forebrain; hb, hindbrain; LC, locus coeruleus; mb, midbrain; NA, noradrenergic neurons. |
|
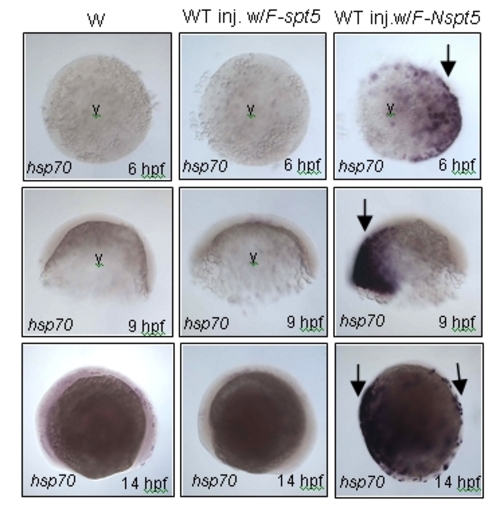
In situ hybridization showing hsp70-4 expression. Increased hsp70-4 expression in WT injected with F-Nspt5 RNA (right, arrows), as compared to WT (left), or WT injected with F-spt5 RNA (middle), at three different developmental stages. Abbreviation: y, yolk. |
|
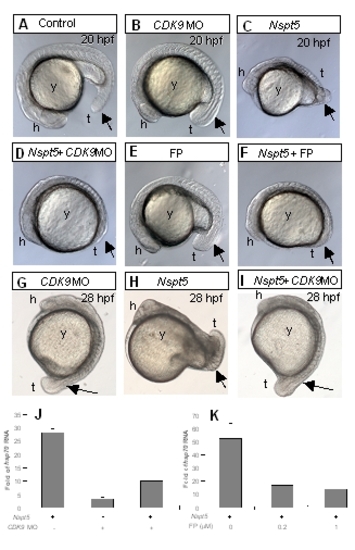
P-TEFb is required for NSpt5’s in vivo effects. (A-D) The morphological phenotypes of CDK9 morpholino-injected (B), NSpt5-expressing (C), or CDK9 morpholino-injected and NSpt5-expressing (D) embryos. (E-F) flavopiridol (FP) treated (E) embryos as compared to FP treated and NSpt5-expressing embryos (F). (G-I) The morphological phenotypes of CDK9 morpholino-injected (G), NSpt5-expressing (H), or CDK9 morpholino-injected and NSpt5-expressing (I) embryos at later developmental stages. These studies show that impairment of CDK9 activity partially suppressed the dorsalization phenotype of NSpt5-expressing embryos. (J-K) Quantitative RT-PCR analyses show that both CDK9 MO and FP treatment decreases hsp70 transcripts induced by NSpt5. Abbreviations, h, head; t, tail; y, yolk. |