- Title
-
A complex extracellular sphingomyelinase of Pseudomonas aeruginosa inhibits angiogenesis by selective cytotoxicity to endothelial cells
- Authors
- Vasil, M.L., Stonehouse, M.J., Vasil, A.I., Wadsworth, S.J., Goldfine, H., Bolcome, R.E. 3rd, and Chan, J.
- Source
- Full text @ PLoS Pathog.
|
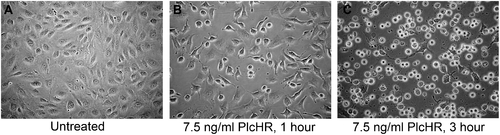
Treatment with pM (PlcHR MW = 95.6 kDA) concentrations of PlcHR induces morphological changes, but not direct lysis of EC. (A) Untreated HUVEC. (B) HUVEC treated with 7.5 ng/ml PlcHR2 for 1 h. (C) HUVEC treated with 7.5 ng/ml PlcHR2 for 3 h. Pictures are at 200x magnification. |
|
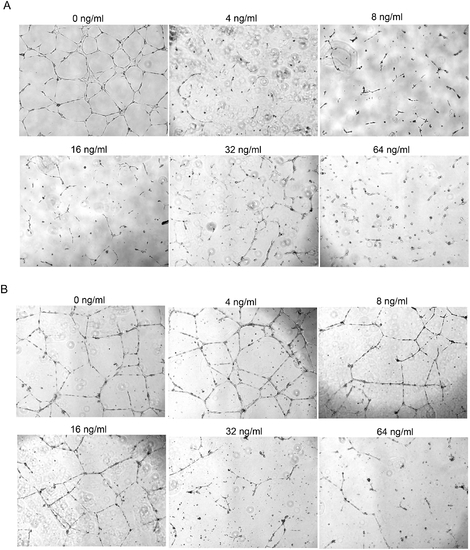
PlcHR inhibits tube formation by EC. (A) EC were challenged with 4–64 ng/ml of PlcHR during tube formation. Tube formation was then measured at 48 h on matrigel matrix. (B) EC that had already formed tubes after 24 h were then subsequently challenged with 4–64 ng/ml of PlcHR for an additional 24 h. Photographs were taken at 40x maginification. |
|
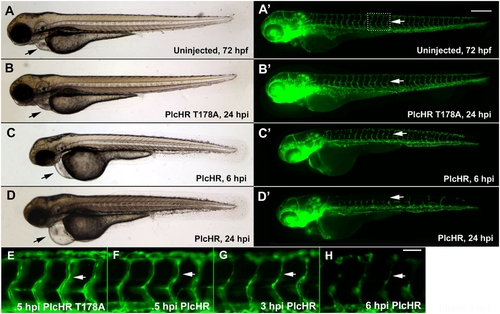
PlcHR causes cardiovascular effects in the zebrafish. Transgenic zebrafish embryos expressing EGFP in endothelial cells, (Tg(Fli:EGFP) [41]) were utilized to examine PlcHR effects. (A–H) Embryos at 48 hpf (hours post-fertilization) were injected with 2 ng of PlcHR, or a catalytically inactive mutant PlcHR T178A, and examined over the next 24 h. PlcHR T178A-injected embryos appeared phenotypically similar to controls (A,B). Prime panels denote visualization of EGFP-labeled endothelial cells in the same embryo. (C) At 6 hpi (hours post-injection), embryos injected with PlcHR had little or no circulation and pericardial edema (black arrows). (D) Effects became more pronounced by 24 hpi as intersegmental vessels regress (ISV, white arrows). (E–H) Higher magnification of the ISVs revealed that their lumens collapsed before endothelial cell regression. Representative pictures of embryos from one of three independent experiments; n>20 per condition. Scale bars indicate 250 μm for (A–D), 50 μm for (E–H). |
|
PlcHR reduces endothelial cell numbers in zebrafish embryos. (A–C) Acridine orange staining was used to indicate cell death at the location of endothelial cells in wildtype embryos injected with 2 ng of PlcHR at 6 hpi (arrows). Corresponding prime panels are enlarged areas showing cell death. (D–H) Zebrafish embryos expressing endothelial nuclear EGFP (Tg(Fli:nucEGFP) [46]) were utilized to assess PlcHR effects on endothelial cell numbers. As before, embryos at 48 hpf were injected with 2 ng of PlcHR, or PlcHR T178A, and compared with uninjected controls. To standardize the region used for counting nuclei, only those in the 8 ISVs immediately anterior to the cloaca were recorded [42]. (D,E,H) At 6, 12, and 24 hpi, there was no significant difference between PlcHR T178A-injected and uninjected animals. (C–F) Those injected with PlcHR showed a clear reduction in endothelial cell numbers by 6 hpi (P<.001). (H) Data taken from three independent experiments; n = 6, statistics performed by t-test. |
|
PlcHR acts on endothelial cells independently of erythrocytes wildtype (Tg(Fli:EGFP)) [44], and GATA1 morphants were injected with PlcHR or PlcHR T178A at 48 hpf and observed for phenotypes (n>20 per condition). PlcHR induced the same effects in morphants as were observed in wild type embryos (C,D), while no effects were noted in either group of embryos when injected with PlcHR T178A (A,B). Prime panels show effects on endothelial cells. (E,F) Depict the lack of circulating blood cells in GATA1 morphants and their presence in wildtype (Tg(fli1:EGFP):Tg(gata1:dsRED)) [44],[45] embryos at the time point in which embryos were scored, respectively. Scale bar indicates 250 μm. |
|
Zebrafish embryos display vascular collapse and then recover over a 24 h time period at low PlcHR doses. Transgenic zebrafish embryos expressing EGFP in endothelial cells and RFP in erythrocytes (Tg(fli1:EGFP):Tg(gata1:dsRED)) [41],[42]) were injected with 1 ng of PlcHR, or its catalytic mutant, as before. (A–F) Single and double prime panels denote visualization of EGFP-labeled endothelial cells or RFP-labeled blood cells in the same embryos, respectively. At 6 hpi, embryos injected with PlcHR displayed little or no circulation (compare D″ with A″, B″). (E–F) Circulation was partially restored by 12 hpi, and embryos recovered by 24 hpi. Representative pictures of embryos from one of three independent experiments; n>20 per condition. White arrow denotes blood flow in dorsal aorta. Scale bar indicates 250 μm. |
|
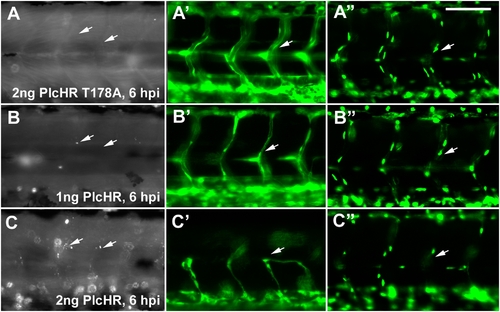
Endothelial cell death does not play a predominant role in zebrafish circulatory collapse and recovery following a 1 ng dose of PlcHR. (Tg(Fli:EGFP) [41]) and (Tg(Fli:nucEGFP) [Roman et al., 2002]) embryos were injected with 1 or 2 ng of PlcHR, or 2 ng of its catalytic mutant as before (A–C). (A–C) Acridine orange staining detected minimal cell death following the injection of 1 ng PlcHR (A′,B′), ISVs collapsed, but did not regress as in the 2 ng dose (C′), and the endothelial nuclei did not decrease [(A″,B″) and data not shown]. (C,C′,C″) The 2 ng PlcHR dose generated increased cell death, vessel regression, and reduced endothelial cell nuclei. Scale bar indicates 100 μm. |