- Title
-
A beta1,4-galactosyltransferase is required for convergent extension movements in zebrafish
- Authors
- Machingo, Q.J., Fritz, A., and Shur, B.D.
- Source
- Full text @ Dev. Biol.
|
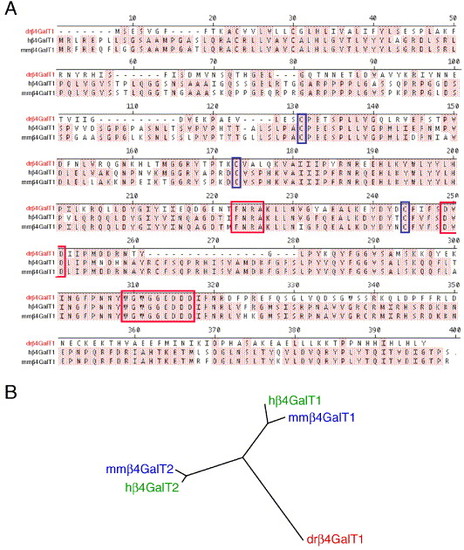
β4GalT1 is a zebrafish ortholog of mammalian β4GalT1. β4GalT1 was identified by in silico homology to human β4GalT1 and was cloned from a 48 h total RNA library. (A) Alignment of β4GalT1 from zebrafish (dr/red) human (h) and mouse (mm). Identical residues are shaded. Predicted catalytic residues are boxed in red, and cysteines used to align the sequences are boxed in blue. (B) Unrooted phylogenetic tree demonstrating the relationship between zebrafish β4GalT1 (red) and human (green) and mouse (blue) β4GalT1 and β4GalT2, suggesting an ancestral relationship. |
|
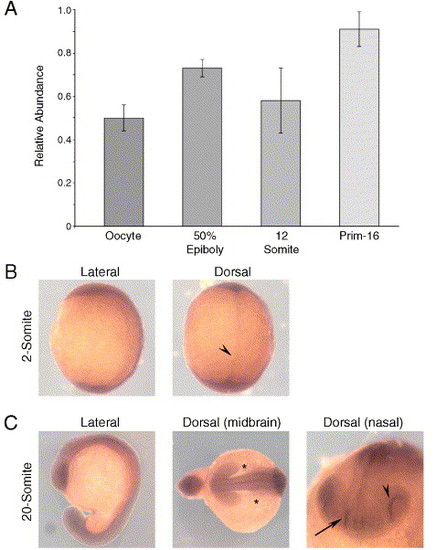
β4GalT1 is expressed throughout the first 24 h of embryonic development. (A) Semi-quantitative RT-PCR for β4GalT1 in staged RNA libraries; oocyte, 50% epiboly, 12-somite and Prim-16. Expression is first detected in the oocyte and remains relatively constant in all stages assayed. Error bars = ±SEM. (B, C) In situ hybridizations for β4GalT1. (B) Two-somite stage, lateral and dorsal views, display β4GalT1 expression throughout the embryo. The increased signal in the midline (arrow) is most likely due to increased tissue mass. (C) 26-somite stage embryos; lateral, dorsal at the midbrain level, and nasal views are shown, demonstrating tissue-specific increases in expression. β4GalT1 expression in the lateral mesoderm ends abruptly at the midbrain level (asterisks) and shows high expression in the developing retina (arrowhead) and nasal placode (arrow). EXPRESSION / LABELING:
|
|
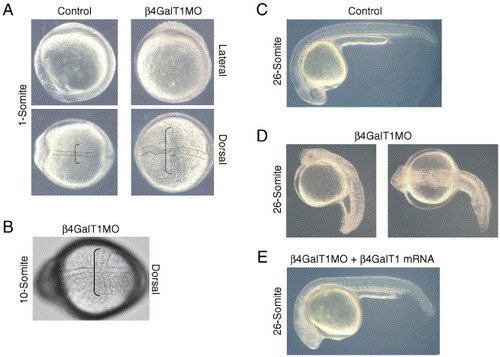
Knockdown of β4GalT1 results in a complex patterning defect. (A) One-somite embryos injected with either control or β4GalT1 morpholino oligonucleotides. In β4GalT1MO embryos, the notochord shows an undulating phenotype (doted lines) and extensively elongated somites (brackets). (B) 10-somite stage embryo illustrating the laterally elongated somites (bracket). (C) 26-somite stage embryo injected with control morpholino oligonucleotides displays a wild-type phenotype. (D) 26-somite stage β4GalT1MO embryos have a complex phenotype, consisting of a truncated anterior–posterior axis, disorganization of the lateral mesoderm, and bending of the tail. (E) Embryos injected with β4GalT1MO2 and an EST encoding the putative catalytic domain of zebrafish β4GalT1 results in a “rescue” of the morphant phenotype. |
|
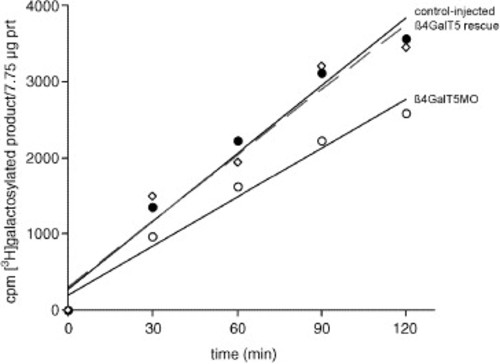
Reduced galactosyltransferase activity in β4GalT1 morphant embryos. Lysates of control-injected (•), morpholino-injected (&Omicron), and morpholino + β4GalT1 mRNA injected (i.e., rescued) (Ê) embryos were assayed for galactosyltransferase activity towards N-acetylglucosamine (GlcNAc). A 30% reduction in activity is seen in lysates from morphant embryos (moderate phenotype at 18 somite stage), relative to lysates from control-injected embryos. Results are representative of three independent assays (two MO1, one MO2). Co-injection of mRNA encoding the catalytic domain of zebrafish β4GalT1 along with MO2 rescued the morphant phenotype (Fig. 3) and brought galactosyltransferase activity back to control levels. The relative decrease in activity (∼30%) should not be taken as an indication of the relative contribution of β4GalT1 to the total repertoire of zebrafish galactosyltransferases, since residual β4GalT1 transcripts likely remain and the relative affinity of the different β4GalT isoforms towards GlcNAc is unknown. |
|
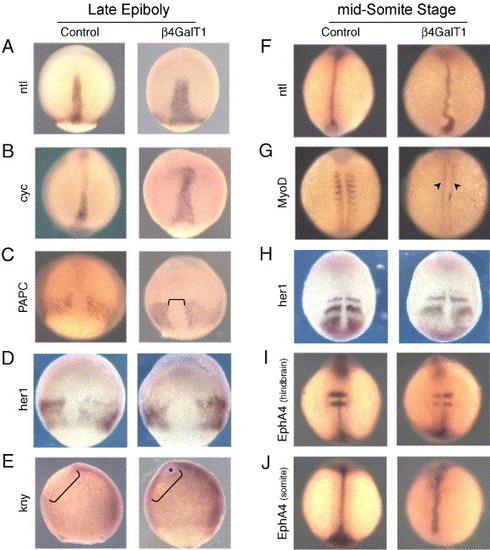
Molecular markers define an early axial patterning defect in β4GalT1 morphant embryos. (A–E) Control and β4GalT1MO embryos at 80–90% epiboly. (F–J) Control and β4GalT1MO embryos at the mid-somite stage. In situ hybridization for ntl (A) and cyc (B) shows shortened and widened expression domains in β4GalT1MO embryos. (C) In situ hybridization for PAPC marks the lateral mesoderm, illustrating a wider and more irregular axial domain (bracket) than in control embryos. (D) her1 in situ hybridizations reveal that fgf signaling is occurring in the lateral mesoderm, but the axial mesoderm interface is irregular, as seen with PAPC. (E) kny expression is retained in the evacuated zone (bracket indicates the evacuated zone) suggesting a defect in convergent extension. (F) ntl expression continues to be truncated in 10-somite β4GalT1MO embryos, and shows severe undulation. (G) MyoD expression is retained in the adaxial cells (arrowheads) of β4GalT1MO embryos, whereas expression in the somitic mesoderm is markedly reduced relative to control embryos. (H–J) Patterning of the somitic mesoderm and central nervous system appear relatively normal in β4GalT1MO embryos as indicated by her1 (H) and EphA4 (I, J) expression, respectively, although the somitic mesoderm is more diffuse and the notochord retains its undulating appearance, as seen with ntl. EXPRESSION / LABELING:
|
|
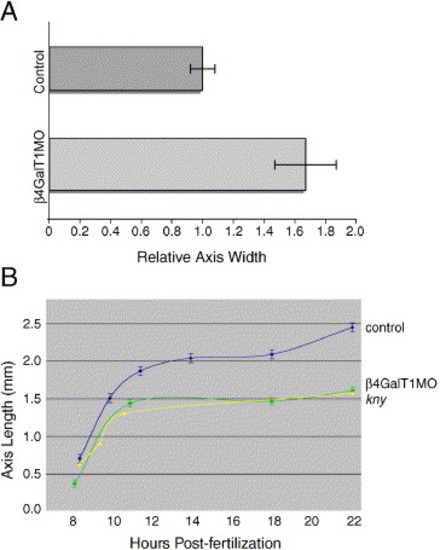
Quantification of abnormal anterior–posterior axis morphology in β4GalT1MO embryos. (A) The width of the dorsal axis was determined at 85% epiboly for both control and β4GalT1MO embryos. The axial width in control embryos was set to 1. The axial width in β4GalT1MO embryos is increased by 63%. Error bars = ±SEM. (B) The rate of anterior–posterior axis elongation was determined for both control and β4GalT1MO embryos. Control embryos (—) undergo a sharp elongation between 8 and 12 h post-fertilization that is also seen in β4GalT1MO embryos (∗); however, the β4GalT1MO embryos are slightly shorter. Following 14 h of development, control embryos undergo a second elongation event, which is absent in β4GalT1MO embryos. These results mimic those reported for classic convergent extension mutations, such as kny (▲) (adapted from Topczewski et al., 2001). |
|
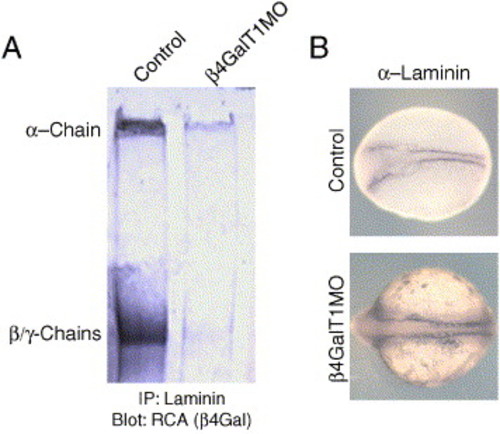
Laminin is improperly galactosylated in β4GalT1 morphant embryos. (A) Control and β4GalT1MO embryos were lysed, and laminin was immunoprecipitated with a-laminin antibodies. The immunoprecipitate was resolved by SDS-PAGE and probed for RCA-I reactivity. Control embryos have strong RCA reactivity at 400 kDa reflecting the α chain of laminin, as well as at 200 kDa, representing the β and γ chains. β4GalT1MO embryos retain only weak RCA reactivity to the 400-kDa protein and almost no RCA reactivity at 200 kDa. (B) To confirm that β4GalT1MO embryos still express laminin, control-injected and β4GalT1-morpholino-injected embryos were stained for laminin. Control embryos (6 somites) have laminin expression along the notochord as well as along the lateral mesoderm of the head. β4GalT1MO embryos (12 somites) retain laminin expression along the notochord, although the domain is wider. |
Reprinted from Developmental Biology, 297(2), Machingo, Q.J., Fritz, A., and Shur, B.D., A beta1,4-galactosyltransferase is required for convergent extension movements in zebrafish, 471-482, Copyright (2006) with permission from Elsevier. Full text @ Dev. Biol.